Aug 28 2009
IBM (NYSE: IBM) scientists have been able to image the "anatomy" -- or chemical structure -- inside a molecule with unprecedented resolution, using a complex technique known as noncontact atomic force microscopy.
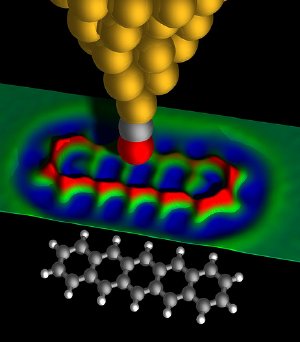 Imaging the "anatomy" of a pentacene molecule - 3D rendered view: By using an atomically sharp metal tip terminated with a carbon monoxide molecule, IBM scientists were able to measure in the short-range regime of forces which allowed them to obtain an image of the inner structure of the molecule. The colored surface represents experimental data. Image courtesy of IBM Research – Zurich
Imaging the "anatomy" of a pentacene molecule - 3D rendered view: By using an atomically sharp metal tip terminated with a carbon monoxide molecule, IBM scientists were able to measure in the short-range regime of forces which allowed them to obtain an image of the inner structure of the molecule. The colored surface represents experimental data. Image courtesy of IBM Research – Zurich
The results push the exploration of using molecules and atoms at the smallest scale and could greatly impact the field of nanotechnology, which seeks to understand and control some of the smallest objects know to mankind.
"Though not an exact comparison, if you think about how a doctor uses an x-ray to image bones and organs inside the human body, we are using the atomic force microscope to image the atomic structures that are the backbones of individual molecules," said IBM Researcher Gerhard Meyer. "Scanning probe techniques offer amazing potential for prototyping complex functional structures and for tailoring and studying their electronic and chemical properties on the atomic scale."
The team’s current publication follows on the heels of another experiment published just two months ago in the June 12 issue of Science (Volume 324, Issue 5933, pp. 1428 – 1431) where IBM scientists measured the charge states of atoms using an AFM. These breakthroughs will open new possibilities for investigating how charge transmits through molecules or molecular networks. Understanding the charge distribution at the atomic scale is essential for building smaller, faster and more energy-efficient computing components than today’s processors and memory devices. These components could one day contribute to IBM's vision of a smarter planet by helping instrument and interconnect the physical world.
As reported in the August 28 issue of Science magazine, IBM Research – Zurich scientists Leo Gross, Fabian Mohn, Nikolaj Moll and Gerhard Meyer, in collaboration with Peter Liljeroth of Utrecht University, used an AFM operated in an ultrahigh vacuum and at very low temperatures ( –268oC or – 451oF) to image the chemical structure of individual pentacene molecules. With their AFM, the IBM scientists, for the first time ever, were able to look through the electron cloud and see the atomic backbone of an individual molecule. While not a direct technological comparison, this is reminiscent of x-rays that pass through soft tissue to enable clear images of bones.
The tip that tipped the scale
The AFM uses a sharp metal tip to measure the tiny forces between the tip and the sample, such as a molecule, to create an image. In the present experiments, the molecule investigated was pentacene. Pentacene is an oblong organic molecule consisting of 22 carbon atoms and 14 hydrogen atoms measuring 1.4 nanometers in length. The spacing between neighboring carbon atoms is only 0.14 nanometers—roughly 1 million times smaller then the diameter of a grain of sand. In the experimental image, the hexagonal shapes of the five carbon rings as well as the carbon atoms in the molecule are clearly resolved. Even the positions of the hydrogen atoms of the molecule can be deduced from the image.
"The key to achieving atomic resolution was an atomically sharp and defined tip apex as well as the very high stability of the system," said IBM scientist Leo Gross. To image the chemical structure of a molecule with an AFM, it is necessary to operate in very close proximity to the molecule. The range, where chemical interactions give significant contributions to the forces, is less than a nanometer. To achieve this, the IBM scientists were required to increase the sensitivity of the tip and overcome a major limitation: Similar to the way two magnets would attract or repel each other when getting close, the molecules would easily be displaced by or attach to the tip when the tip was approached too closely—rendering further measurements impossible.
Gross added, "We prepared our tip by deliberately picking up single atoms and molecules and showed that it is the foremost tip atom or molecule that governs the contrast and resolution of our AFM measurements." A tip terminated with a carbon monoxide (CO) molecule yielded the optimum contrast at a tip height of approximately 0.5 nanometers above the molecule being imaged and—acting like a powerful magnifying glass—resolved the individual atoms within the pentacene molecule, revealing its exact atomic-scale chemical structure.
Furthermore, the scientists were able to derive a complete three-dimensional force map of the molecule investigated. "To obtain a complete force map the microscope needed to be highly stable, both mechanically and thermally, to ensure that both the tip of the AFM and the molecule remained unaltered during the more than 20 hours of data acquisition," says Fabian Mohn, who is working on his Ph.D. thesis at IBM Research – Zurich.
To corroborate the experimental findings and gain further insight into the exact nature of the imaging mechanism, IBM scientist Nikolaj Moll performed first-principles density functional theory calculations of the system investigated. He explains, "The calculations helped us understand what caused the atomic contrast. In fact, we found that its source was Pauli repulsion between the CO and the pentacene molecule." This repulsive force stems from a quantum mechanical effect called the Pauli exclusion principle. It states that two identical electrons can not approach each other too closely.
The scientific paper entitled "The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy" by L. Gross, F. Mohn, N. Moll, P. Liljeroth, and G. Meyer, appears in Science, Volume 325, Issue 5944, pp. 1110 – 1114 (28 August 2009).
IBM Scientists First to Image the Anatomy of a Molecule