Dec 11 2018
Electron microscopy is one of the most extensively used techniques for inspecting protein structure. The analysis of these structures is extremely important to describe their function, feeding fundamental data into several fields like cancer research, cell biology, structural biology, and a host of other biomedical fields. In addition, it also provides a better understanding of biomineralization.
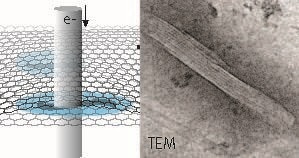 Schematic representation of hydrated microtubule proteins encapsulated between two graphene layers imaged by transmission electron microscopy (TEM). An example of a TEM image of a microtubule is shown at the right The interior lining reflects the protofilament structure of the polymeric microtubule. Free within this press release. (Image credit: INM, Niels de Jonge)
Schematic representation of hydrated microtubule proteins encapsulated between two graphene layers imaged by transmission electron microscopy (TEM). An example of a TEM image of a microtubule is shown at the right The interior lining reflects the protofilament structure of the polymeric microtubule. Free within this press release. (Image credit: INM, Niels de Jonge)
Liquid-phase electron microscopy, or LPEM, is a novel option for imaging proteins. This technology has the ability to image native, or unstained, protein structure and other samples like cells or nanomaterials in liquid. LPEM was developed over the past 15 years. Until now, it debated whether the liquid samples’ radiation tolerance would be worse or better as opposed to amorphous ice.
Sercan Keskin and Niels de Jonge from the INM-Leibniz Institute for New Materials have now shown in their recent publication that the radiation tolerance is elevated by an order of magnitude when compared to a sample in ice. This outcome was accomplished by preparing a sample of microtubules in a graphene liquid cell. It was important to use the lowest possible speed at which the electron beam irradiation was applied.
Usually, a metal is used to fix and stain samples to improve their contrast, and these samples are then dried, incorporated in plastic, sliced in thin sections, and finally imaged in the vacuum environment needed for electron microscopy. The disadvantages related to this sample preparation are overcome by cryo-electron microscopy, which offers a way to examine proteins in a close to native-hydrated state by formulating them in amorphous ice.
Conversely, the samples are highly sensitive to electron beam irradiation, which presents a major limitation. As a result, statistical noise present in the image prevents high resolution, and several thousand noisy images of analogous structures have to be imaged to resolve the structure.