| The semi permeable lipid bilayer cell membrane separates different ion concentrations (charges) on the inner and outer side of the membrane. Therefore, the cell membrane has the electrical properties of a plate capacitor (CM). The electrochemical gradient results in a membrane potential that can be measured directly with an intracellular electrode. When ion channels are opened due to chemical or electrical stimulation, the corresponding ions are moved along their electrochemical gradient. In other words, the resistance (RM) of the membrane is lowered, resulting in an inward or outward flow of ions, measured as a transmembrane current. Conductivity Levels of the Extracellular Space in Cell Membranes The extracellular space is conductive as well, and though the resistance is very low, it is not zero. According to Ohm's law (V=RxI, where V=voltage, R=resistance, and I=current), the extracellular current results in a small voltage that can be measured with extracellular electrodes. Extracellular Signals Extracellular signals are smaller than transmembrane potentials, depending on the distance of the signal source to the electrode. Due to the low-pass filtering properties of the extracellular space, extracellular signal amplitudes decrease with increasing distance of the signal source to the electrode. Therefore, a high spatial resolution of the electrode array and/or a close interface between electrode and cell membrane is very important for a high signal-to-noise ratio. The transmembrane current and the extracellular potential follow the same time course, and are roughly equal to the first derivative of the transmembrane potential. Industry Applications for Non-Invasive Extracellular Recording Devices Over the last 30 years, non-invasive extracellular recording from multiple electrodes has developed into a widely-used standard method. Expert-driven advice in the field of neuroscience, pharmacology, analog and digital electronic design, have led to high-end products in basic science and pharmaceutical industry. Systems and methods have been greatly improved, leading to more features, lower costs, and higher throughput. Almost all excitable or electrogenic cells and tissues can be used for extracellular recording in vitro, for example, central or peripheral neurons, heart cells, retina, or muscle cells. What is a Microelectrode Array? A microelectrode array (MEA) is an arrangement of several (typically more than 60) electrodes allowing the targeting of several sites for stimulation and extracellular recording at once. A complete extracellular recording system can be summarized by the following components: • Signal source (cells / tissue), • Cell / sensor interface, • Biosensor (MEA), • Filter amplifier, • Recording hardware and software. What are Microelectrode Arrays (MEAs) Used for? MEAs can be used to examine the activities of whole cells and tissues rather than single receptors (as in patch clamp experiments), studying the interaction of several cells in a culture or in their natural environment or even in whole organs. Furthermore, MEAs allow us to study the spatio-temporal pattern of responses in a neuronal network or slice by using a new class of information, not only about time and amplitude, but also about space, in a two-dimensional setup (creating spatial maps). The Lab-On-A-Chip Project Based on 3C-SiC Technology The emerging field of monitoring biological signals generated during nerve excitation, synaptic transmission, quantal release of molecules and cell-to-cell communication, stimulates the development of new methodologies and materials for novel applications of bio-devices in basic science, laboratory analysis and therapeutic treatments. We have planned the realisation of four activities with the following tasks: Task 1. Development of new biocompatible substrates favoring neuronal growth along specific pathways. Task 2. Monitoring of electrical activity from neuronal networks. Task 3. Resolution of cellular excitability over membrane micro areas. Task 4. Detection of quantal released molecules by means of newly designed biosensors. Growing 3C-SiC Films on to Silicon Substrates Task number 1 has been realized by means of SiC substrates, by plating the cells directly on the substrate or eventually with an additional proteic layer. For this aim, thanks to the long term experience acquired, 3C-SiC films with controlled stoichiometry, different thickness and crystalline quality are grown directly on silicon substrates or on silicon substrates previously ‘carbonised’. Using the Low Pressure Chemical Vapor Deposition (LPCVD) Growth Process The growth technique used is an LPCVD (Low Pressure Chemical Vapor Deposition - heater temperature up to 2000°C), whose main advantage is represented by its high ‘deposition rate’. Primary cultures of hippocampal neurons or neurosecretory cells from the adrenal medulla and insulin-secreting cells have been used as experimental models. Besides monitoring cell survival and adhesion on the SiC substrate, the goal of these experiments is to check the maintenance of functional properties such as cellular excitability and secretion: these properties are monitored, at this stage of the project, with classical electrophysiological approaches and compared with the well-reported electrical activity of cells plated on routinely used plastic dishes. | 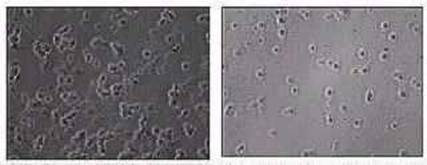
| | Figure 1. Different nucleation of neuron cultures grown on polycrystalline and amorphous silicon carbide. | Producing SiC Microelectrode Arrays (MEAs) Whose Dimensions are Compatible with the Cellular Soma The main objective of task number 2 is the realization of SiC microelectrode arrays whose dimensions will be compatible with the cellular soma (10-20 um). In this structure, every element of the array is constituted by a doped 3C-SiC region, with metallic interconnections coated with amorphous silicon carbide, so that silicon carbide represents the only material interfaced to the biological environment. This initial prototype of microelectrode array should allow the monitoring of synaptic transmission either between pre- and postsynaptic terminals of two neurons forming mono-synapses, or between neurons forming multiple synapses in complex neuronal networks. The possibility of simultaneously recording electrical signals from several neurons without affecting the cell interior represents the greater innovation of this project, with respect to conventional electrophysiological approaches using glass microelectrodes. The Benefits of Using Solid-State Microelectrode Arrays The classical methods require sophisticated and expensive micro-movements for electrode positioning, which are limited to 2 or 3 units per recording units and produce irreversible damage to the cell interior. This later drastically limits the duration of the recording and does not allow the repetition of the recording over the same cell with time. Solid-state microelectrode arrays allow us to overcome these drawbacks. It is possible in fact to: 1. Record electrical signals with good signal-to-noise ratios without perturbing the intracellular cell content, 2. Monitor simultaneously the activity of various neurons belonging to a complex network, 3. Repeat periodically the recordings on the same cells over long period of times. Improving Silicon Carbide (SiC) Arrays by Using Microelectrodes For the realization of Task number 3, the SiC array will be improved by constructing microelectrodes in the submicrometric range, in order to reveal electrical signals from different areas of the same cell. This part of the project will be fundamental for quickly approaching some still debated points concerning the distribution of voltage-gated ion channels (Na+, Ca2+ and K+) in specialized areas of the membrane, or the preferential involvement of some channel subtypes in the mechanism of excitation-secretion coupling. Building a SiC-Electrodes Array as a Chemical Detector for Oxidizable Molecules Released During Cell Activity The objective of task number 4 is the construction of a prototype of SiC-electrodes array as a chemical detector for oxidizable molecules released during cell activity triggered by chemical substances (KCl or acetylcholine) on chromaffin cells of the adrenal gland. In this case every element of the array (a 10 um wide square) will host four electrodes placed at the corners of the square. Respect to classical electrochemical methods, requiring polarized carbon fibers with rough dimensions of 10 micrometers in diameter, the SiC multielectrode array should greatly improve the monitoring of secretory vesicles fusion to the plasma-membrane, allowing the spatial localization and temporal resolution of the event. The possibility of simultaneously recording exocytotic processes from different areas of the same cell would in fact allow to resolve the spatial distribution of vesicles inside secretory cells and study the kinetics of vesicle fusion and neurotransmitter release. Catecholamines secreting cells (chromaffin cells of the adrenal gland) will be the experimental model used in this task, for the greater dimension of their secretory granules (200-300 nm diameter) respect to the synaptic vesicles (60-80 nm) and for the high concentration of stored neurotransmitter (~1 M noradrenaline). Note: A complete list of references can be found by referring to the original text. |