Many samples when prepared for zeta potential measurement will sediment because of their size or density.
This raises two subjects for discussion, firstly, does sedimentation during the measurement affect the zeta potential and secondly, does the change in the size of the particles during the measurement affect the result.
What is Zeta Potential?
The fundamental principle of the measurement of zeta potential using the Malvern Panalytical Zetasizer, is the measurement of the velocity of particles undergoing electrophoresis. The velocity of these particles, or mobility when expressed in unit field, is converted to the zeta potential using the Henry equation. (Ref. 1)
How is Zeta Potential Measured?
Particles dispersed in a liquid are introduced into a horizontal cell with electrodes at either end. When a potential is applied to the electrodes, particles with a negative zeta potential will migrate towards the positive electrode and vice-versa. The velocity of the particles is measured using laser Doppler electrophoresis.
The optics for the velocity measurement are arranged so as only to be sensitive to movement in the horizontal direction.
Effect of Sedimentation on Particle Size Distribution
Samples prepared by grinding, micronising or precipitation will have a broad size distribution. This means that as the sample sediments, the size of particles remaining in the measurement volume will decrease with time as the smaller particles sediment more slowly.
The Effect of Sedimentation on Zeta Potential
The zeta potential is actually unaffected by this size change. This is because the number of charges on the particle surface driving the particle forward, and the viscous drag on the particle, are both proportional to the surface area. As the average particle size decreases, the viscous drag reduces proportionally. These changes cancel out any size dependence of the measurement.
Results
Graph 1 (below) shows the results of measurement of calcium carbonate dispersed in dilute pH7 buffer.
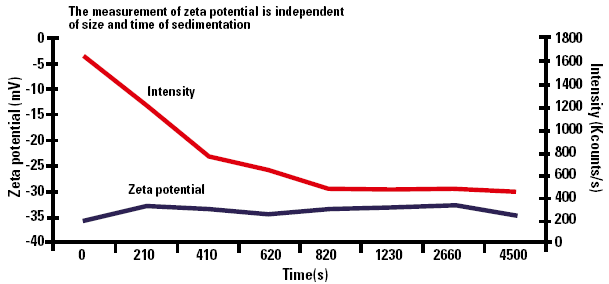
Figure 1. Zeta potential as a function of sedimentation time.
This ensures that any movement in the vertical direction, due to sedimentation for example, will not affect the measurement.
The measurement of zeta potential is independent of size and time of sedimentation. The scattered light intensity shows a decrease with time which is due to the material sedimenting out of the scattering volume of the cell.
The graph shows that the zeta potential changes by only ±1.5mV over the measurement period of more than an hour.
Conclusions
For samples like dispersions of calcium carbonate, neither sedimentation during measurement, nor the consequent change in size of the particles measured, affect the zeta potential.
References
1. Hunter, R.J. Zeta potential in colloid science (1981) Academic Press ISBN 0-12-361961.
Source: "The Study of Antibody: Antigen Interactions Using Light Scattering Techniques", Application Note by Malvern Panalytical.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information please visit Malvern Panalytical.