|
The reduction of metal salts using tetraalkylammonium-hydrotriorganoborates in organic solvents yields metal colloids stabilized by NR4+. The metal particles are well protected by long chain alkyl groups, which make the colloid very soluble in lipophilic organic phases giving up to one molar solutions of zerovalent metals:
MXv + vNR4(BEt3H) → Mcolloid + vNR4X + vBEt3 + v/2 H2
M = metals of the Group 6 – 11 of the Periodic Table; X = Cl, Br;v = 1, 2, 3; R = alkyl, C6 – C20
Resultant Particle Sizes
This very versatile classic method yields generally monodisperse nanoparticulate organosols of 2 – 3nm size, e.g. Cr (2 – 3nm), Mo (2.5nm), Fe (3nm), Co (2.8nm), Ni (2.8nm), Rh (2.1nm), Pd (2.5nm), and Pt (2.8nm). Relatively small particles are found in case of Ru (1.3nm) and Ir (1.5nm). In the coin metal series larger particle sizes are produced with this procedure, e.g. Cu (8.3nm), Ag (8 – 13nm), and Au (10nm).
Purification
Typically the organosols are isolated as viscous, waxy materials which contain ca. 4 – 10 wt-% of metal. The protecting shell can, however, be washed away to increase the metal content of the sol. For example, a raw Pt organosol containing 4 – 6 wt.-% of Pt can be “purified” by redispersion in ether and subsequent precipitation with ethanol.
After drying a greyish-black Nano-Pt-colloid is obtained (size: 2.8nm ± 0.5nm) which contains 70 – 90 wt-% of Pt depending on the degree of washing. Similarly, a N(Octyl)4Cl-stabilised rhodium organosol (size: 2.1nm ± 0.5nm) may be “purified” to give a greyish-black nano-Rh-colloid containing 73 wt.-% of Rh.
Via co-reduction of the corresponding metal salts, a 1.7nm ± 0.5nm bimetallic N(Octyl)4Cl-stabilised Pt/Ru colloid ( 6.9 wt.-% Pt; 3.6 wt.-% Ru) is formed which serves as a valuable precursor to fuel cell anode catalysts. A small sized N(Octyl)4Br-stabilised gold colloid (2.6nm ± 1.1nm) is available from the thermal decomposition of HAu(NO3)4 x 3 H2O in the presence of N(Octyl)4Br. This material is a good precursor to nanostructured gold and alloy catalysts and electrocatalysts.
The controlled decomposition of low valent complexes, preferably in the presence of stabilizers, yields very clean nanoparticulate precursors for fuel cell catalysts and may be applied also for the decoration or coating of preformed metal cores. It is, however, restricted to the availability of appropriate “starting complexes” (see Fig. 1).
|

|
|
Figure 1. Organosols via hydrogenolysis and thermolysis of organometallic complexes.
|
Production of Organosols by Controlled Complex Decomposition
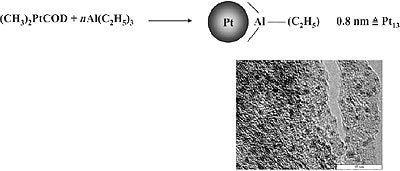
A special case of organosol preparation via controlled complex decomposition is the recently found decomposition of (CH3)2PtCOD in the presence of trialkylaluminum. As shown in Fig. 2, extremely small Pt particles (size 0.8nm) are formed which represent the first “full shell cluster” of Pt having 13 Pt atoms. Remarkably, the protecting shell of these organosol type consists exclusively of alkylaluminum which can be easily removed by washing. On the exposure to air the alkylaluminum shell is oxidized to give a Al2O3 matrix where the small Pt particles are regularly distributed as shown in the High Resolution TEM Micrograph (Fig. 2).
|
|
|
Figure 2. Formation of 0.8nm Pt stabilized by trialkylaluminum.
|
Stabilization
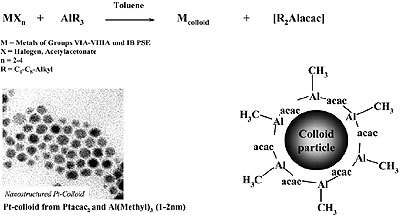
Organoaluminum compounds have been used for the “reductive stabilization” of mono- and bimetallic nanoparticles to give organometallic organosols as shown in Fig. 3.
|
|
|
Figure 3. Reductive stabilization of organosols (e.g. 1 – 2 nm Pt) with trialkylaluminum.
|
Structure and Properties
According to Fig. 3, colloids of zerovalent elements of Groups 6 – 11 of the Periodic Table, and also of tin, may be prepared in the form of stable, isolable organosols. Available analytical data suggest that a layer of condensed organoaluminum species protects the transition metal core against aggregation, as depicted in Fig. 3. However, the exact “backbone” of the colloidal organoaluminum protecting agent has not yet been completely established.
Unreacted organoaluminum groups (e.g. Al-CH3, Al-C2H5) from the starting material are still present in the stabilizer and have been detected by quantitative protonolysis experiments.
A complete list of references can be found by referring to the original document.
|