The availability of particle detectors which can be used at up to 4x10-4 Torr pressures has made it possible to use mass spectrometers at pressures close to those used in several plasma processing systems. This results in better sampling of both neutral and ionized species from plasma reactors.
In addition, the Hiden Analytical quadrupole mass spectrometer (QMS) can be operated in a mode called Threshold Ionisation Mass Spectrometry (TIMS). This mode allows variation in the energy of the electrons emitted within the ionization.
Different elements have defined ionization energies — the energy required to remove one of its orbiting electrons. This energy is based on the electron orbital, where the electrons in the outer shell usually have weaker ionization energies due having lower electrostatic forces and being further away from the positively charged nucleus. Figure 1 shows the resulting electron impact ionization efficiency curves.
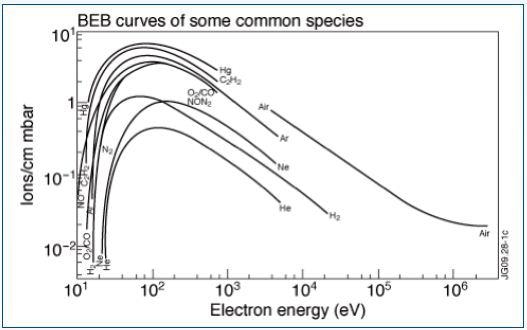
Figure 1. Electron impact ionization efficiency curves
When impacting electrons have a specific minimum energy, the ionization process of neutral particles commences. As minimum energy is based on and specific to any species available in the gas matrix, a spectral “identifier” or fingerprint can be ascertained for all atomic or molecular species.
For example, a particular application of the TIMS technique has been applied to neutral species to precisely determine the helium (He)/deuterium (D2) ratios during plasma fusion wherein the by-product is helium ash.
However, while using a QMS in standard mass spectral mode, this quantification is avoided as the convoluted mass spectral signatures of both D2 and He overlap at 4amu. The electron energy spectra measured using the Hiden Analytical QMS in TIMS mode, for deuterium and Helium with ionization onsets at 15.4eV and 24.5eV, respectively, are shown in Figure 2.
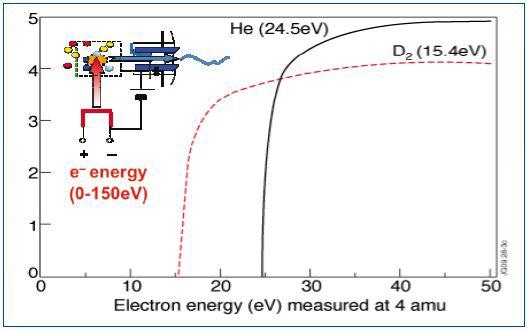
Figure 2. Electron energy spectra for deuterium and helium
The electron energy spectrum resulting from the presence of both the gases is shown in Figure 2a. It is evident from the results that a clear deconvolution of the two species in the TIMS spectra is achieved, allowing accurate detection of D2 in Helium down to ppm detection levels. Hiden Analytical TIMS equipped mass spectrometers are frequently used and presently employed at JET the Joint European Torus experimental nuclear fusion facility, Oxford, UK.
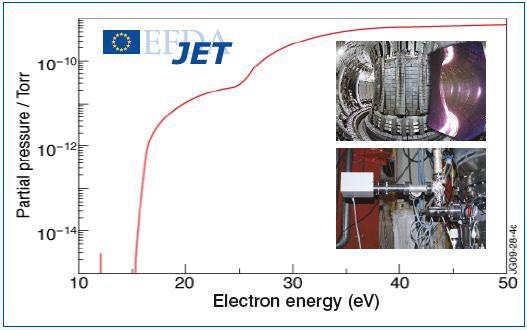
Figure 2a. Electron energy spectrum obtained in presence of deuterium and helium
Experimental Results
Helium has an ionization potential of 24.6eV. The section AB of the curve is a result of the formation of metastable He*m atoms with a long lifetime against spontaneous decay. As they collide with the detector, these atoms gain adequate energy to produce pulse counts.
The section BC of the curve has both ionized and metastable helium contributions for electron energies over 24.6eV. Similar results were obtained for other experiments carried out using argon, krypton and neon.
Figure 3 shows the data for krypton. The curves illustrated in Figure 3 can be interpreted with reference to Figure 4. The schematic system shown in Figure 5 was used to obtain data of Figure 3.
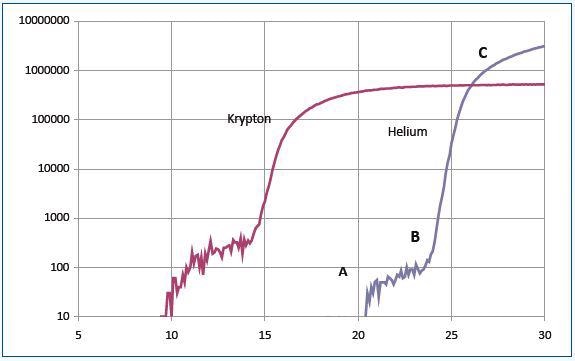
Figure 3. Data for krypton
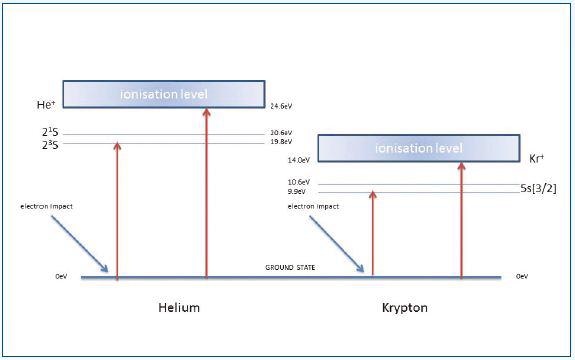
Figure 4. Reference to interpret the form of curves shown in Figure 3
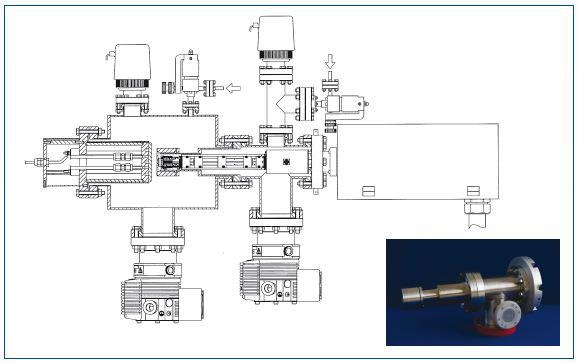
Figure 5. Schematic of the system used to acquire data shown in Figure 4
An RF plasma can be placed in the middle of an electrode and the sampling orifice of the mass spectrometer. The entry of ions from the reactor into the Hiden mass spectrometer can be controlled by the electrodes behind the orifice. The particle detector can be operated at pressures of 4.10-4Torr. Gases were either directly introduced into the mass spectrometer source or into the reactor.
The detector recorded the entry of He*m produced in the plasma under the conditions where the internal ionization source of the mass spectrometer was turned off, plasma operation is carried out in helium, sampling system was set to block all plasma ions from entering it.
As shown in Figure 6, the metastable signal varies proportionally to the gas pressure in the reactor and the plasma power. No energetic particles were observed from the plasma upon replacing the helium plasma with oxygen plasma. This is due to the inadequate energy of the long-lived metastable oxygen species to be recorded by the detector.
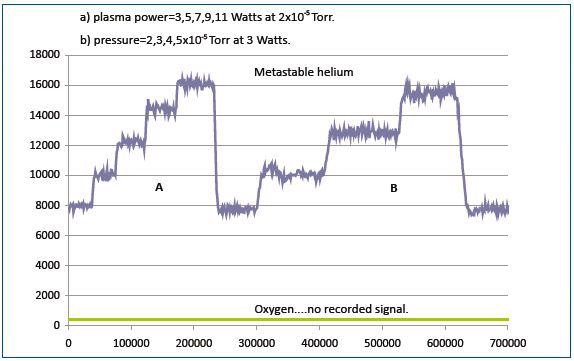
Figure 6. The metastable signal is proportionalto the gas pressure in the reactor and the plasma power
Under the operating conditions of both plasma and mass spectrometer, the sampling system was again set to block plasma ions. Figure 7 shows the recorded signals for a mixture of helium and oxygen. The section BC for the helium curve shows the ions generated from ground-state helium sampled from the reactor.
The section AB shows ions generated from sampled metastable helium. Metastable helium atoms generated in the source between 20 and 25eV have a minor contribution. The threshold energy (not shown) is likely to be around 5eV. Penning ionization was not observed in the internal source for oxygen.
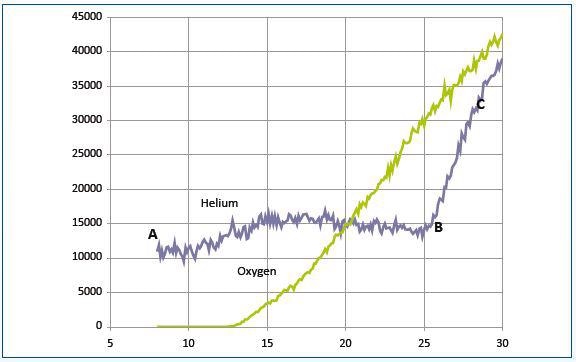
Figure 7. The recorded signals for a mixture of helium and oxygen
Figure 8 shows the data for pure oxygen obtained under the conditions of 15W plasma at 30mTorr and a mass spectrometer source pressure of 2.10-4Torr. Two components with onset potential difference of about 1eV were appeared to be in the region below 16eV.
This condition can be expected when the sampled oxygen includes metastable a 1Δg oxygen or when the latter was generated in the mass spectrometer source. The present experiment is influenced by the source process.
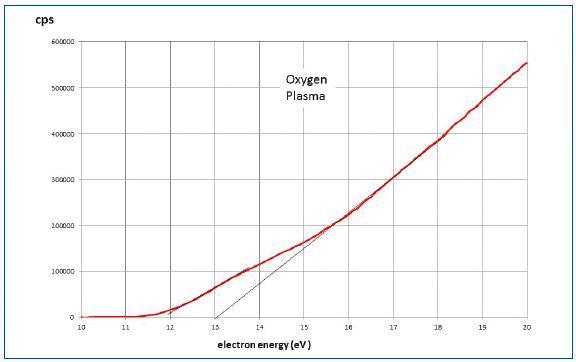
Figure 8. Data for pure oxygen under condition of 15W plasma at 30mTorr and a mass spectrometer source pressure of 2.10-4Torr
Conclusion
When the metastable species produced in the plasma possess adequate internal energy and long life-times, they can be directly detected by reducing the pressure differential between an attached mass spectrometer and a plasma reactor.
In this experiment, the process of detecting low energy, yet long-lived, metastable species and other plasma products has been simplified. These measurements may help in assessing the role of energetic neutral species in the plasma processing of surfaces.

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.