In this article, we discuss research in the field of green nanotechnology, published in the journal Biotechnology and Bioprocess Engineering. Here, zinc oxide nanocomposites (ZnO NPs) were synthesized using pericarp of pomegranate and chemical precipitation. The prepared nanocomposites exhibited decent antimicrobial and antioxidant activity, indicating their potential for biomedical applications.

Image Credit: George Dolgikh/Shutterstock.com
Within the flourishing field of nanotechnology are nanoparticles (NPs)—particles sized about 100 nm. NPs are classified into four types: carbon, polymer, metal, and metal-oxide, and other inorganic substances.
Due to their low toxicity, metal-oxide NPs are widely used, with ZnO NPs employed in numerous applications, including modern drug delivery systems. They are also used as external antibacterial agents in the preparation of ointments, food packaging, lotion, cloth fabrics, and mouthwash to avoid microbial load.
ZnO NPs, in particular, often feature in cosmetics to safeguard the skin from harmful radiations, and the Food and Drug Administration (FDA, USA) accepts ZnO as a GRAS (Generally Recognized As Safe) material.
The cosmetic industry has increasingly relied on plant-derived natural compounds, mainly plant extracts having antibacterial, antioxidant, and photoprotective effects. Previous research confirmed that ZnO NPs could be synthesized using orange fruit and peel extracts for antibacterial activities. Furthermore, beneficial phytochemicals, like flavonoids, alkaloids, carotenoids, terpenoids, and tannins of fruits can be used as nanocomposite agents to synthesize nanoparticle materials.
Punica granatum L. (Pomegranate), an edible fruit belonging to the family Punicaceae (now in the Lythraceae family), is rich in natural polyphenols, flavonoids, and proanthocyanidins. The pericarp of Pomegranate fruit contains potent bioactive compounds like gallagic, ellagitannins, ellagic acid, punicalagin, pelargonidin, anthocyanins delphinidin, and luteolin.
Below details the methodology utilized to synthesize novel PE-ZnO NPs with pomegranate ethanol extract (PE), and subsequent analyses for antioxidant, antibacterial and cytotoxicity properties in vitro using human keratinocyte cell lines.
Methodology
Chemicals
Folin-Ciocalteu, 2, 2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 1,1-diphenyl-2-picryl hydrazyl (DPPH), and Methyl thiazolyl diphenyl-tetrazolium bromide (MTT), fetal bovine serum (FBS), trypsin-disodium ethylenediamine tetra-acetic acid (EDTA), Dulbecco's modified Eagle's medium (DMEM) were used along with phosphate-buffered saline (PBS) and penicillin (100 units/mL)/streptomycin (100 μg/mL).
Ethanol Extract of Pomegranate Pericarp
The healthy pomegranate fruits were harvested and washed. The pericarps were cut and dried at room temperature before being ground using an electronic grinder to a coarse powder and stored in an incubator at 4 °C. An extract was prepared, and the pooled pomegranate extract (PE) was then used for the analysis of phenolics, antioxidant, and antibacterial activities.
Determination of Total Polyphenolic and Flavonoid Content
The collected PE was examined for the total phenolic content and total flavonoid content.
Synthesis and Characterization of PE-ZnO NPs
Pomegranate-pericarp extract functionalized zinc oxide nanocomposite (PE-ZnO NPs) was synthesized using the chemical precipitation method and the NPs were dried in the incubator at 40 °C.
The synthesized PE-ZnO NPs were analyzed for the nanocomposite formation by monitoring the metallic plasmonic peaks in the UV-Vis spectra calculated using a UV/Vis spectrophotometer and their morphologies were examined using Scanning Electron Microscope (SEM).
ABTS+ and DPPH Scavenging Activity Measurement
PE-ZnO NPs’ antioxidant activity was analyzed by ABTS·+ free radical scavenging method. The DPPH free radical scavenging activity was also assessed for the PE-ZnO NPs.
Cell Culture
Human keratinocytes (HaCaT) cell line was grown and maintained in Dulbecco’s Modified Eagle Medium (DMEM) and subculture was performed at 2–3 days intervals.
Cytotoxicity and Proliferation Test
MTT assay was used to analyze the cytotoxicity and proliferation inhibition induced by the synthesized PE-ZnO NPs.
Antimicrobial Activity
Bacterial strains, i.e., Bacillus licheniformis (ATCC 1458), Bacillus cereus (ATCC 14579), and Escherichia coli (ATCC 15597) were used for the antibacterial activity assay against Tetracycline as a positive control.
Statistical Analysis
All data were calculated as Mean ± S.D values using the SPSS package (SPSS, USA).
Results and Discussion
Pomegranate pericarp extracts functionalized ZnO NPs (PE-ZnO NPs) were synthesized, characterized, and examined for biological properties. Profiling of the bioactive compounds showed a considerable percentage of polyphenols and flavonoids in PE, which can be used to synthesize PE-ZnO NPs.
Total Polyphenolic Content Assay of PE Extracts
The pericarp of pomegranate, which is typically discarded, was evaluated for the total bioactive content and is employed in the synthesis of PE-ZnO NPs. The PE revealed substantial quantities of polyphenol and flavonoid (Table 1).
Table 1. Total polyphenolic and flavonoids contents of Punica granatum extraction. Source: Singh, et al., 2021.
| Extracts |
Total Polyphenol
(mg GAE#/extract g) |
Total flavonoid
(mg QE##/extract g) |
| Punica granatum |
363.04 ± 0.10a |
20.17 ± 0.01a |
#GAE: gallic acid equivalents; ##QE: quercetin equivalents. aMean ± SD (n = 3).
Physical properties of PE-ZnO NPs
PE-ZnO NPs were synthesized via a chemical precipitation method and the nanocomposite material formation was analyzed using UV-Vis spectra (Figure 1A).
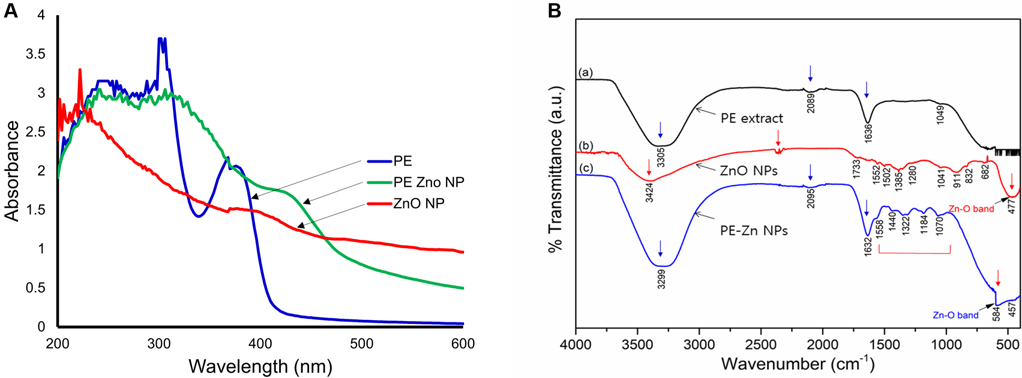
Figure 1. (A) UV absorption spectrum of PE, ZnO NPs, and PE-ZnO NPs. (B) The FT-IR spectra analysis of PE, ZnO NPs, and PE-ZnNP. Image Credit: Singh, et al., 2021.
FT-IR spectroscopy was used to pinpoint the functional groups related to PE, ZnO NPs, and PE-ZnO NPs (Figure 1B).
Depending on FT-IR results, functionalization of PE with ZnO NPs was established. SEM imaging characterized the developed nanostructures in PE-ZnO NPs in comparison to ZnO NPs. The ZnO NPs were sheets and foamlike nanostructures (Figure 2A and 2B) while the PE-ZnO NPs were clusters of nanoplates (Figure 2C and 2D).
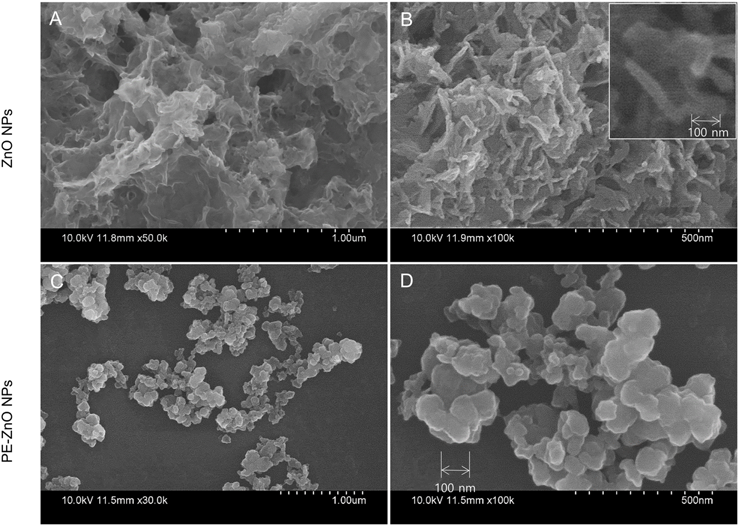
Figure 2. (A and B) This is a schematic diagram showing the shape of ZnO NPs. (C and D) Pomegranate-zinc oxide nanoparticles (PEZnO NPs) were measured by SEM. Image Credit: Singh, et al., 2021.
In vitro Antioxidant Activity of PE and PE-ZnO NPs
PE and PE-ZnO NPs showed enhanced ABTS+ and DPPH radical scavenging activity in a concentration-dependent fashion. The percentage scavenging activity of PE and PE-ZnO NPs (1,000 μg/mL) were 95.1% and 95.2%, respectively (Figure 3A and 3B).
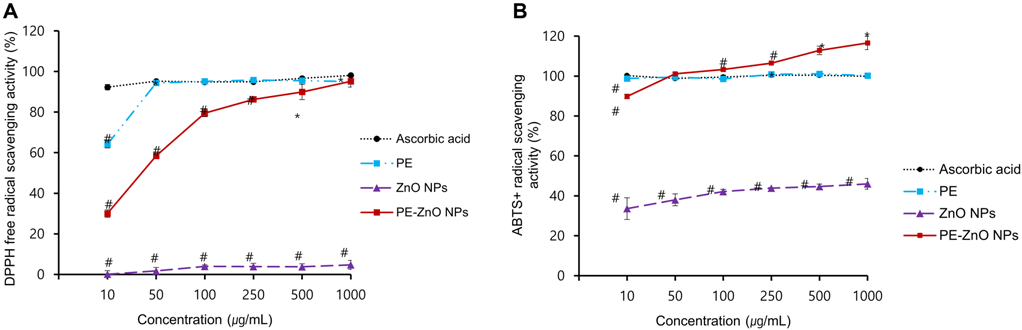
Figure 3. (A and B) DPPH and ABTS·+ radical scavenging activity of PE, ZnO NPs, and ZnO-PE NPs. Results are the means ± SD, * p < 0.05, # p < 0.005 compared with ascorbic acid (AA). Image Credit: Singh, et al., 2021.
Cytotoxicity and Proliferation Rate of PE-ZnO NPs in HaCaT Cells
Cytotoxicity activity was analyzed using MTT assay to investigate the nontoxic concentration of PE-ZnO NPs in HaCaT cells line. Results demonstrated that that PE and PE-ZnO NPs do not show significant toxicity to HaCaT cells (Figure 4).
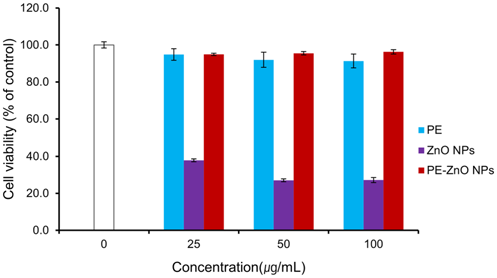
Figure 4. Effect of PE, ZnO NPs, and PE-ZnO NPs on human skin epithelial keratinocytes (HaCaT) cells viability. The cells were treated with different concentrations of PE-ZnO NPs (25, 50, and 100 µg/mL) for 24 hours and then assessed for cell viability by MTT assay. Image Credit: Singh, et al., 2021.
In vitro Antimicrobial Activity of PE-ZnO NPs
The antibacterial activity of PE and PE-ZnO NPs tested at various percentages against B. cereus, B. licheniformis, and E. coli by colony-forming unit (CFU) showed that the treatments with PE-ZnO NPs (1.0 mg/mL) showed a wide range of antimicrobial activities (Figure 5).
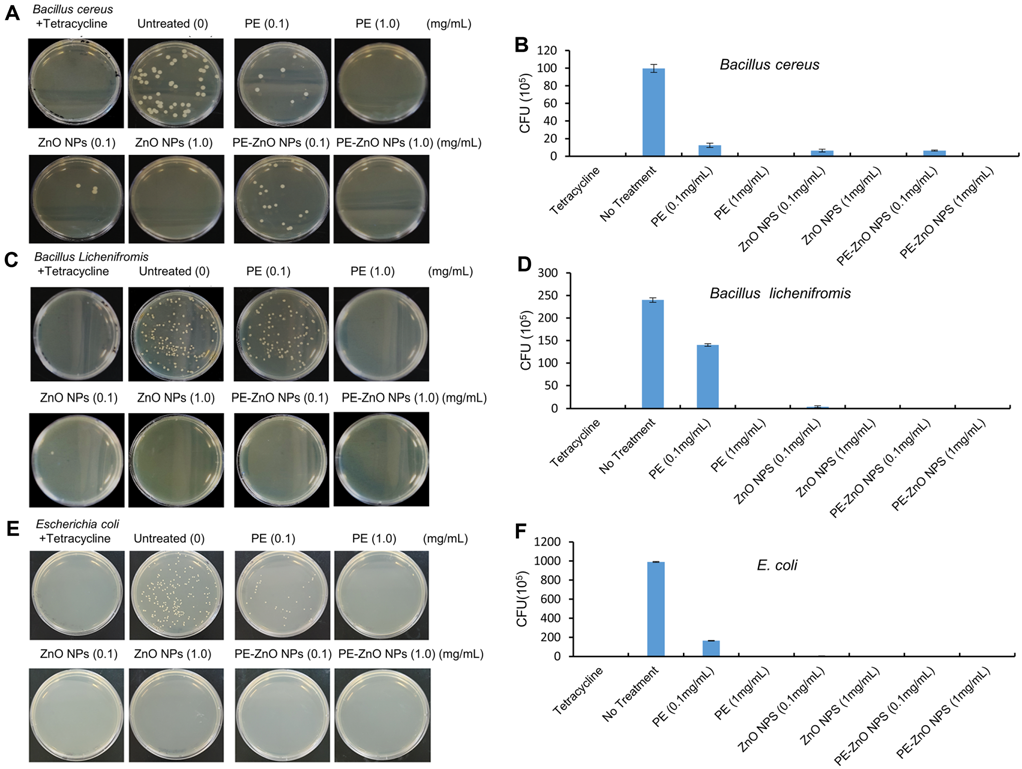
Figure 5. The antibacterial efficiency of the PE, ZnO NPs, and PE-ZnO NPs against Bacillus cereus, Bacillus licheniformis, and Escherichia coli. The colony-forming units (CFU/mL) of PE, ZnO NPs, and PE-ZnO NPs. Image Credit: Singh, et al., 2021.
Conclusion
ZnO NPs synthesized by functionalizing the bioactive compounds in the pomegranate pericarp ethanol extracts exhibited strong radical scavenging and antimicrobial activity. The results of this research indicate that these organic metal-based nanoparticles are a valuable nanomaterial, with potential applications as multi-target antimicrobial agents in pharmaceutical and cosmetics.
Continue reading: Nanotechnology and Green Technology: How Can the Two Work Hand in Hand?
Journal Reference:
Singh, M., Lee, K. E., Vinayagam, R., Kang, S. G. (2021) Antioxidant and Antibacterial Profiling of Pomegranate-pericarp Extract Functionalized-zinc Oxide Nanocomposite. Biotechnology and Bioprocess Engineering, 26, pp. 728–737. Available at: https://link.springer.com/article/10.1007%2Fs12257-021-0211-1.
References and Further Reading
- Sirelkhatim, A., et al. (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Letters, 7, pp. 219–242. doi.org/10.1007/s40820-015-0040-x.
- Ali, A., et al. (2018) Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnology Reviews, 7, pp. 413–441. doi.org/10.1515/ntrev-2018-0067.
- Długosz, O., et al. (2020) Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine. Materials, 13, p. 279. doi.org/10.3390/ma13020279.
- Jiang, J., et al. (2018) The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorganic chemistry and applications, 2018, p. 1062562. doi.org/10.1155/2018/1062562.
- Patra, J. K., et al. (2018) Nano based drug delivery systems: recent developments and future prospects. Journal of Nanobiotechnology, 16, p. 71. doi.org/10.1186/s12951-018-0392-8.
- Gao, Y., et al. (2019) Biofabrication of zinc oxide nanoparticles from Aspergillus niger, their antioxidant, antimicrobial and anticancer activity. Journal of Cluster Science, 30, pp. 937–946. doi.org/10.1007/s10876-019-01551-6.
- Román, L. E., et al. (2019) Blocking erythemally weighted UV radiation using cotton fabrics functionalized with ZnO nanoparticles in situ. Applied Surface Science, 469, pp. 204–212. doi.org/10.1016/j.apsusc.2018.11.047.
- Srinivasan, S., et al. (2020) Biogenic metal nanoparticles and their antimicrobial properties. In: S. B. Dhull, P. Chawla, and R. Kaushik (eds.). Nanotechnological Approaches in Food Microbiology. CRC Press, Boca Raton, FL, USA.
- Siddiqi, K. S., et al. (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Research Letters, 13, p. 141. doi.org/10.1186/s11671-018-2532-3.
- Włodarczyk, R & Kwarciak-Kozłowska, A (2021) Nanoparticles from the cosmetics and medical industries in legal and environmental aspects. Sustainability, 13, p. 5805. doi.org/10.3390/su13115805.
- Jeevanandam, J., et al. (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein Journal of Nanotechnology, 9, pp. 1050–1074.
- Katz, L. M., et al. (2015) Nanotechnology in cosmetics. Food and Chemical Toxicology, 85, pp. 127–137. doi.org/10.1016/j.fct.2015.06.020.
- Smijs, T G & Pavel, S (2011) Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnology, Science and Applications, 4, pp. 95–112. doi.org/10.2147%2FNSA.S19419.
- Twilley, D., et al. (2021) Ethanolic extracts of South African plants, Buddleja saligna Willd. and Helichrysum odoratissimum (L.) Sweet, as multifunctional ingredients in sunscreen formulations. South African Journal of Botany, 137, pp. 171–182. doi.org/10.1016/j.sajb.2020.10.010.
- Thi, T. U. D., et al. (2020) Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Advances, 10, pp. 23899–23907. doi.org/10.1039/D0RA04926C.
- Luque, P. A., et al. (2018) Green synthesis of zinc oxide nanoparticles using Citrus sinensis extract. Journal of Materials Science: Materials in Electronics, 29, pp. 9764–9770. doi.org/10.1007/s10854-018-9015-2.
- Nava, O. J., et al. (2017) Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. Journal of Molecular Structure, 1147, pp. 1–6. doi.org/10.1016/j.molstruc.2017.06.078.
- Rajendran, N. K., et al. (2021) Synthesis of zinc oxide nanoparticles using Rubus fairholmianus root extract and their activity against pathogenic bacteria. Molecules. 26, p. 3029. doi.org/10.3390/molecules26103029.
- Qin, G., et al. (2017) The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. The Plant Journal, 91, pp. 1108–1128. doi.org/10.1111/tpj.13625.
- Fahmy, H., et al. (2020) Pomegranate juice as a functional food: a comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food and Function, 11, pp. 5768–5781. doi.org/10.1039/D0FO01251C.
- Viuda-Martos, M., et al. (2010) Pomegranate and its many functional components as related to human health: a review. Comprehensive Reviews in Food Science and Food Safety, 9, pp. 635–654.
- Fischer, U. A., et al. (2011) Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chemistry, 127, pp. 807–821. doi.org/10.1016/j.foodchem.2010.12.156.
- Caruso, A., et al. (2020) Pomegranate: nutraceutical with promising benefits on human health. Applied Sciences, 10, p. 6915. doi.org/10.3390/app10196915.
- Khajebishak, Y., et al. (2019) Punicic acid: A potential compound of pomegranate seed oil in Type 2 diabetes mellitus management. Journal of Cellular Physiology, 234, pp. 2112–2120. doi.org/10.1002/jcp.27556.
- Xu, K. Z. Y., et al. (2009) Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. Journal of Ethnopharmacology, 123, pp. 280–287. doi.org/10.1016/j.jep.2009.03.035.
- Stowe, C. B. (2011) The effects of pomegranate juice consumption on blood pressure and cardiovascular health. Complementary Therapies in Clinical Practice, 17, pp. 113–115. doi.org/10.1016/j.ctcp.2010.09.004.
- Aviram, M., et al. (2002) Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans. Drugs under Experimental and Clinical Research, 28, pp. 49–62.
- Lansky, E P & Newman, R A (2007) Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. Journal of Ethnopharmacology, 109, pp. 177–206. doi.org/10.1016/j.jep.2006.09.006.
- Larrosa, M., et al. (2010) Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. The Journal of Nutritional Biochemistry, 21, pp. 717–725. doi.org/10.1016/j.jnutbio.2009.04.012.
- Wang, L & Martins-Green, M (2014) Pomegranate and its components as alternative treatment for prostate cancer. International Journal of Molecular Sciences, 15, pp. 14949–14966. doi.org/10.3390/ijms150914949.
- Hajleh, M A & Al-Dujaili, E A S (2016) Anti-cancer activity of pomegranate and its biophenols; general review. EC Nutrition, 6, pp. 28–52.
- Howell, A B & D’Souza, D H (2013) The pomegranate: effects on bacteria and viruses that influence human health. Evidence Based Complementary Alternate Medicine, 2013, p. 606212. doi.org/10.1155/2013/606212.
- Khaleghnezhad, V., et al. (2019) Interactive effects of abscisic acid and temperature on rosmarinic acid, total phenolic compounds, anthocyanin, carotenoid and flavonoid content of dragonhead (Dracocephalum moldavica L.). Scientia Horticulturae, 250, pp. 302–309. doi.org/10.1016/j.scienta.2019.02.057.
- Re, R., et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26, pp. 1231–1237. doi.org/10.1016/S0891-5849(98)00315-3.
- Blois, M. S. (1958) Antioxidant determinations by the use of a stable free radical. Nature, 181, pp. 1199–1200. doi.org/10.1038/1811199a0.
- Kandylis, P & Kokkinomagoulos, E (2020) Food applications and potential health benefits of pomegranate and its derivatives. Foods, 9, p. 122. doi.org/10.3390/foods9020122.
- Akhtar, S., et al. (2015) Pomegranate peel and peel extracts: Chemistry and food features. Food Chemistry, 174, pp. 417–425. doi.org/10.1016/j.foodchem.2014.11.035.
- Derakhshan, Z., et al. (2018) Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food and Chemical Toxicology, 114, pp. 108–111. doi.org/10.1016/j.fct.2018.02.023.
- Ardekani, M. R. S., et al. (2011) Comparative antioxidant activity and total flavonoid content of Persian pomegranate (Punica granatum L.) cultivars. Iranian Journal of Pharmaceutical Research, 10, pp. 519–524.
- Del Buono, D., et al. (2021) Biogenic ZnO nanoparticles synthesized using a novel plant extract: Application to enhance physiological and biochemical traits in maize. Nanomaterials, 11, p. 1270. doi.org/10.3390/nano11051270.
- Senthilkumar, N., et al. (2017) Synthesis of ZnO nanoparticles using leaf extract of Tectona grandis (L.) and their antibacterial, anti-arthritic, antioxidant and in vitro cytotoxicity activities. New Journal of Chemistry, 41, pp. 10347–10356. doi.org/10.1039/C7NJ02664A.
- Sundrarajan, M., et al. (2015) Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Advanced Powder Technology, 26, pp. 1294–1299. doi.org/10.1016/j.apt.2015.07.001.
- Fu, L & Fu, Z (2015) Plectranthus amboinicus leaf extract-assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceramics International, 41, pp. 2492–2496. doi.org/10.1016/j.ceramint.2014.10.069.
- Ambika, S & Sundrarajan, M (2015) Green biosynthesis of ZnO nanoparticles using Vitex negundo L. extract: spectroscopic investigation of interaction between ZnO nanoparticles and human serum albumin. Journal of Photochemistry and Photobiology B: Biology, 149, pp. 143–148. doi.org/10.1016/j.jphotobiol.2015.05.004.
- Sowndhararajan, K & Kang, S C (2013) Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi Journal of Biological Sciences, 20, pp. 319–325. doi.org/10.1016/j.sjbs.2012.12.005.
- Liao, C., et al. (2020) Interactions of zinc oxide nanostructures with mammalian cells: Cytotoxicity and photocatalytic toxicity. International Journal of Molecular Sciences, 21, p. 6305. doi.org/10.3390/ijms21176305.
- Chmielewska, A & Szajewska, H (2010) Systematic review of randomised controlled trials: probiotics for functional constipation. World Journal of Gastroenterology, 16, pp. 69–75.
- Song, D., et al. (2012) Recent application of probiotics in food and agricultural science. In: E. Rigobelo (ed.). Probiotics. IntechOpen.
- Stenfors A. L. P., et al. (2008) From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiology Reviews, 32, pp. 579–606. doi.org/10.1111/j.1574-6976.2008.00112.x.
- Haydushka, I. A., (2012) Recurrent sepsis due to Bacillus licheniformis. Journal of Global Infectious Diseases, 4, pp. 82–83. doi.org/10.4103%2F0974-777X.93768.
- Croxen, M. A., et al. (2013) Recent advances in understanding enteric pathogenic Escherichia coli. Clinical Microbiology Reviews, 26, pp. 822–880. doi.org/10.1128/CMR.00022-13.
- Pelfrene, E., et al. (2021) Antimicrobial multidrug resistance in the era of COVID-19: a forgotten plight? Antimicrobial Resistance & Infection Control, 10, p. 21. doi.org/10.1186/s13756-021-00893-z.