A team of researchers recently published a paper in the journal Scientific Reports that evaluated the effectiveness of commercial remineralizing topical formulations/ mousses and toothpaste in the remineralization of demineralized dental tissues.
Study: Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulation. Image Credit: Maxx-Studio/Shutterstock.com
Importance of Remineralization of Dental Tissues
Demineralization represents the outcome of extremely complex chemical and biological interactions that take place at the interface of the dental hard tissues, the biofilm colonizing the hard tissues, and the external oral environment, and the phenomenon is responsible for dental erosion and caries. Enamel demineralization primarily occurs when the pH value of the oral microenvironment reaches below the critical pH of dental tissues.
Hydroxyapatite (HA), a major component of dental enamel, is soluble in acidic solutions as it has a critical pH of 5.5. Any pH value that is below the critical pH value in an oral environment can lead to the gradual dissolution of hard tissues. However, the demineralization can be reversed when there is sufficient availability of phosphate and calcium ions and the pH values are higher than seven in the oral microenvironment.
In these conditions, the phosphate and calcium ions precipitate and cover the dental surfaces with an amorphous mineral layer, which acts as a precursor to an organized mineral structure, leading to remineralization. The phenomenon is referred to as epitaxial growth.
Although the buffering capacity and salivary ion content in physiological conditions can compensate for the enamel demineralization process by creating an equilibrium between the remineralization and demineralization phases, certain changes in the oral microenvironment, such as substantial carbohydrate intake, can disrupt this equilibrium and accelerate demineralization. Thus, an external supply of phosphate and calcium ions is necessary to facilitate remineralization.
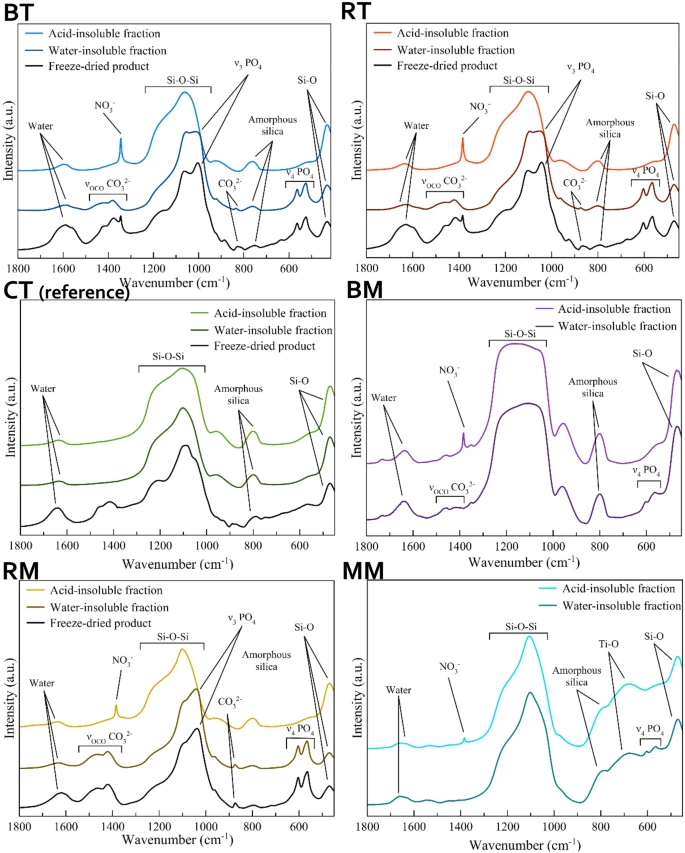
Figure 1. FT-IR spectra of the tested formulations. © Ionescu, A.C., Degli Esposti, L., Iafisco, M. et al. (2022)
Existing External Ways to Remineralize the Demineralized Dental Tissues
Topical formulations and toothpaste represent a reliable and effective way to deliver these ions to dental hard tissues. Thus, it has now become a standard practice to include remineralizing compounds in mousses and toothpaste.
Fluoride is the most effective among these compounds in inhibiting the demineralization process as fluoride ion possesses several anti-caries properties.
However, calcium phosphate-based systems such as synthetic HA and amorphous calcium phosphate (ACP) particles, specifically nanoparticles, can also promote the remineralization processes. Moreover, the solubility, orientation, growth, and nucleation of synthetic HA crystals can be modulated by doping HA with fluoride, carbonate strontium, and zinc ions.
Both ACP and doped HA were successfully used as active remineralizing ingredients in several mousses and toothpaste, with many of these products containing calcium phosphate phases in form of stabilized nanoparticles.

Figure 2. Representative micrographs at different magnifications (2000 × , 10,000 × , and 20,000 ×) of the enamel surface after treatment with the tested and reference formulations, compared to the negative control (demineralized surface stored in PBS). © Ionescu, A.C., Degli Esposti, L., Iafisco, M. et al. (2022)
Evaluation of Remineralizing Mousses and Toothpaste
In this study, researchers compared the physical and chemical composition, along with in vitro bioremineralization activity of five commercially available topical formulations and toothpaste containing casein phosphopeptides (CPP)-ACP or ion-substituted HA. Traditional fluoride-containing toothpaste was used as a reference for the study. Researchers also correlated the composition of studied formulations with their role in remineralization.
MI Paste Plus (MM), Biorepair desensitizer enamel repair (RM), and Biosmalto Caries Protection (BM) were the mousse, while Biorepair Plus (RT) and Biosmalto caries abrasion and erosion (BT) were the toothpaste used in the study. Colgate triple action (CT) was utilized as a reference formulation. Samples representing a water-insoluble fraction and an acid-insoluble fraction of each toothpaste and mousse were prepared for the study.
Fourier transform infrared (FT-IR) spectroscopy and powder X-ray diffraction (PXRD) was used for the structural characterization of the prepared samples. Researchers also performed a compositional analysis of both whole products and prepared samples and in vitro evaluation of dentinal tubules occlusion and remineralization.
Research Findings
All toothpaste contained 40–50 weight percent water, 20–40 weight percent water-soluble matter, and 15–20 weight percent water-insoluble inorganic matter. RT and BT contained an acid-soluble inorganic phase, while acid-insoluble titanium dioxide and silicon dioxide were observed in CT.
In topical formulations, the lowest water-soluble matter content and highest water content were observed in RM, while BM contained the highest content of water-soluble matter and the lowest amount of water. Additionally, the calcium phosphate content in BM was lower than in RM. MM was composed of a low amount of water-insoluble components and 95% of water and water-soluble ingredients.
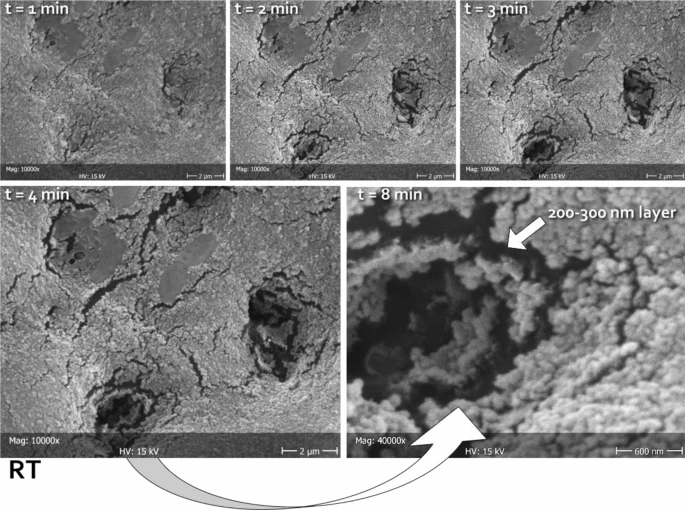
Figure 3. Representative micrographs of the same area (in this case belonging to RT), scanned at different times (1, 2, 3, 4, and 8 min) focusing the electron beam on the opening of tubules, thus heating the carbon-rich collagen structures underneath and disrupting the mineralized material obliterating the lumen. In this way, it was possible to measure the thickness of the deposited layer. The latter can be clearly seen as constituted by a homogeneous assembly of spherical nanoparticles. The absence of exposed HA prisms in this substratum did not allow for epitaxial growth; therefore, no HA nanocrystals could be identified independently from the tested formulations. Considering the treatment time that was allowed in this study (one week), it can be speculated that the tested formulations might need to incorporate a scaffold in order to be able to grow organized structures on the dentinal substrate. © Ionescu, A.C., Degli Esposti, L., Iafisco, M. et al. (2022)
The observations of the water-insoluble phase of RT and BT revealed a high material similarity to biogenic HA and poor crystallinity of both kinds of toothpaste, while the water-insoluble BM and RM indicated that RM contained a higher HA content compared to BM, and its HA was highly crystalline in nature. Carbonate doping of HA in all formulations was confirmed in the FTIR spectra analysis.
The concentration of overall strontium, zinc, fluoride, and magnesium ion content in the whole products and their water-insoluble fractions were almost similar to the values reported in the ingredients list.
All toothpaste and topical formulations containing calcium phosphate bioactive nanocompounds demonstrated their remineralizing ability by epitaxial growth of a layer that displayed the composition and morphology of human HA, which is in contrast to the conventional fluoride-containing toothpaste. Moreover, the presence of CPP or doping ions such as carbonate, fluoride, magnesium, and strontium further facilitated the remineralization process.
Topical formulations displayed a greater tubules occlusion capability compared to toothpaste irrespective of their composition.
Taken together, the findings of this study demonstrated the possibility of effectively restoring the demineralized dental tissues in a biomimetic way through mousse and toothpaste.
Reference
Ionescu, A.C., Degli Esposti, L., Iafisco, M. et al. (2022) Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Scientific Report. https://www.nature.com/articles/s41598-022-09787-5.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.