Mar 2 2009
Large, perfect diamonds are precious to almost all of us but to some scientists, it is the defects that really matter. This is because defects can form nanoscopic color centers, which play a key role in the development of both quantum computing and quantum cryptography. A research team at the Max Planck Institute for Biophysical Chemistry in Göttingen has now probed these color centers inside the crystal with unprecedented resolution using an optical microscope. Using STED microscopy, the scientists identified even densely packed color centers and determined their position inside the crystal with a precision better than 0.15 nanometers, corresponding to the dimension of an atom. (Nature Photonics, 22nd February 2009).
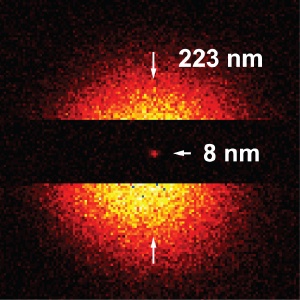 Sharp focus: Recordings of lattice defects in diamond crystals are 28 times sharper with the super resolving STED microscope than with conventional fluorescence microscopy methods, namely 8 nanometers. (Picture: Rittweger & Hell, MPIbpc)
Sharp focus: Recordings of lattice defects in diamond crystals are 28 times sharper with the super resolving STED microscope than with conventional fluorescence microscopy methods, namely 8 nanometers. (Picture: Rittweger & Hell, MPIbpc)
Diamonds are brilliant not only as gem stones but scientists are also increasingly interested in these precious crystals. As the perfect jewel, the colorless variant glitters brilliantly - but in science it is the much cheaper fluorescent diamonds that cause the sensation. Their color comes from impurities, such as nitrogen atoms, in the diamond lattice. If a nitrogen atom sits next to a vacancy in the crystal lattice, a luminescent defect of atomic size is formed. Electrons of these color centers can - similar to dye molecules - be excited by laser light. When they return to the ground state, the excitation energy is converted to fluorescence light. This fluorescence glowing and their ability to form atomically small magnets render color centers in diamond extremely interesting.
Researchers hope to use diamond color centers as small processors in quantum computing to speed up specific arithmetic operations, and their suitability for encoding highly sensitive data is currently being explored. However, there is a crucial drawback for observing these color centers inside the crystal: single defects can only be recognized with a fluorescence microscope, but only if they are further apart than approximately 200 nanometers (millionth of a millimeter) because this is the resolution limit of a standard optical microscope.
Stefan Hell's group at the Max Planck Institute for Biophysical Chemistry in Göttingen succeeded in recording the first images of densely packed individual color centers employing STED (Stimulated emission depletion) microscopy. They pushed the current resolution limit of STED down to a few nanometers. Diamond color centers closer than a tiny fraction of the resolution limit could be separated and their position determined with a precision of 0.15 nanometers. Scientists have now a method at hand to individually address densely packed color centers - with conventional lenses and focused light. For the ongoing research and application of these color centers this is a major breakthrough. This work is also important for the field of crystallography, which now has another method at hand to study crystal structures locally.
That nitrogen-vacancies fluoresce after excitation with laser pulses also makes them attractive for a different research field: biological fluorescence nanoscopy. Scientists plan to reveal a live cell's secrets using fluorescent diamonds, requiring tiny diamond nano particles which can be used for labeling cells."Organic fluorescent dyes, which we routinely use for STED, have the disadvantage that they blink and eventually bleach", says Eva Rittweger, a PhD student in the department. "However, color centers in diamonds are extremely photostable even when observed for hours in the STED microscope."
Research groups in Würzburg, Stuttgart, as well as in Asia and America are working on applying the nanocrystals to biological and medical fundamental research. "If we are successful in exploiting this property in nanodiamonds, one would have a new class of fluorescent markers and a form of fluorescence nanoscopy without bleaching. This could, in combination with the nanometric resolution of STED microscopy, improve imaging of the cell at the nanoscale", says Stefan Hell.