Sep 7 2009
Mitsuharu Tabuchi and Tomonari Takeuchi, the Advanced Battery Research Group, the Research Institute for Ubiquitous Energy Devices of the National Institute of Advanced Industrial Science and Technology (AIST), Junji Akimoto, the Crystal Materials Engineering Group, the Advanced Manufacturing Research Institute of AIST, and Junichi Imaizumi, the Analysis Team, Research and Development Department of Tanaka Chemical Corporation have jointly developed two types of cobalt-free oxides, that can be used as cathode materials for lithium ion secondary batteries. Approximately 20% of the total amount of transition metals in the oxide materials is iron, which is abundant and inexpensive.
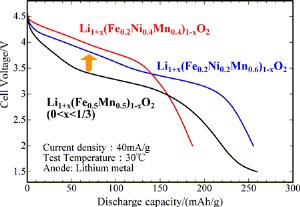 Initial discharge curves of the cathode materials after charging to 5 V: newly developed (red and blue) and preciously developed (black)
Initial discharge curves of the cathode materials after charging to 5 V: newly developed (red and blue) and preciously developed (black)
These materials do not contain cobalt (a rare metal). Despite their high iron content, their discharge voltages of 3.5–3.7 V are higher than the discharge voltage (3.0 V) of Li1+x(Fe0.5Mn0.5)1-xO2, which was previously developed by AIST, and are closer to the discharge voltage (4 V) of the conventional cathode materials such as LiNi1/3Mn1/3Co1/3O2 and LiNi1/2Mn1/2O2. The use of iron is advantageous since it is abundant and reduces costs; however, it used be difficult to develop oxides containing iron as cathode materials. The successful use of iron will help conserve resources and reduce the costs of lithium ion secondary batteries for electric vehicles including hybrid cars.
The details of this study will be published in the 50th Battery Symposium in Japan held at Kyoto International Conference Center, from November 30 to December 2, 2009. The study was conducted as part of a sponsored research project, "Development of battery systems for next-generation vehicles (Li-EAD project)– R & D of novel and high-capacity positive electrode material consists of low-cost constituent oxides (from FY2007 to FY2009)," funded by New Energy and Industrial Technology Development Organization (NEDO).