Our attempts to use solar energy continue to be very ineffective; the true masters of this craft are photosynthetic plants, algae, and bacteria. Science is trying to emulate these organisms.
Igor Nabiev from the NanoGUNE Research Centre in San Sebastian (Spain), Alexander O. Govorov of Ohio University (USA), John Donegan of CRANN, Trinity College Dublin (Ireland), and a team of Spanish, Irish, French, and Russian scientists have now developed a new approach to increasing light efficiency. As they report in the journal Angewandte Chemie, they successfully equipped the photosynthetic center from a purple bacterium with a "light-harvesting antenna" consisting of a quantum dot-an inorganic nanocrystal.
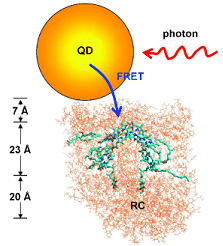
In organisms, the first step of photosynthesis is the absorption of light by an antenna, a complex of proteins and pigments that is brought into an electronically excited state by light energy. The energy packet can then be passed on to special chlorophyll cofactors in the reaction center of the photosynthetic apparatus. There the energy is finally used to produce cellular energy stores such as ATP. The passing-on of the energy packets occurs through a special radiation-free process called Förster resonance energy transfer (FRET), in which the electronic states of the sender and receiver of the energy packets must be brought into resonance.
Artificial photosynthetic systems also require an antenna for the efficient harvesting of light. The antenna must also be able to pass the energy packets along through FRET. Previous synthetic antennas were organic dye molecules, which have the disadvantage of capturing too small a range of wavelengths from sunlight.
Furthermore, they are not stable under long-term irradiation. The new idea in this case was to replace the organic molecules with fluorescing inorganic quantum dots as antennas. Quantum dots are nanoscopic crystals that are so tiny that in many respects they behave like molecules rather than as macroscopic solid objects. The electronic and optical properties of quantum dots, including the wavelengths that they absorb, can largely be made to order, because these are dependent on the size, shape, and composition of the dot. The researchers chose to use quantum dots made of cadmium telluride and cadmium selenide, which fluoresce under irradiation while remaining stable in the long term. The size and surface composition were selected so that they could absorb a particularly broad range of sunlight.
The researchers were able to couple the quantum-dot antenna to a reaction center from the photosynthetic system of a purple bacterium. Under irradiation, the quantum dots then no longer fluoresce; instead they pass the absorbed energy over to the reaction center through FRET. This new approach may clear a path toward novel synthetic photosynthetic systems.