A research team at the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory has developed bilayered nanocrystals of a metal-metal oxide that features multiple catalytic locations on nanocrystal interfaces. The sites enable multiple, sequential catalytic responses to be carried out both selectively and concurrently.
Peidong Yang at Berkeley Lab's Materials Sciences Division, and the University of California Berkeley's Chemistry Department and Department of Materials Science and Engineering, says this development will help design high-performance, multifunctional nanostructured catalysts for multiple-step chemical reactions.
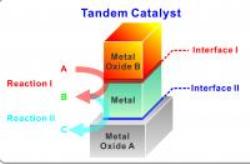 Berkeley's Bilayer Nanocatalyst System
Berkeley's Bilayer Nanocatalyst System
Yang, who led the team, is the co-author of the research paper ‘Nanocrystal bilayer for tandem catalysis’ published in the journal Nature Chemistry. Yusuke Yamada, Chia-Kuang Tsung, Wenyu Huang, Ziyang Huo, Susan Habas, Tetsuro Soejima, Cesar Aliaga and Gabor Somorjai have co-authored the paper.
The team used the Lamgnuir-Blodgett assembly technique to deposit platinum and cerium oxide nanocube monolayers on a silica substrate. The layers measured less than 10nm and piled up to create two discernible metal–metal oxide interfaces of platinum-silica and cerium oxide-platinum. The cerium oxide-platinum interface catalyzed methanol to emit carbon monoxide and hydrogen. These byproducts underwent ethylene hydroformylation through a response catalyzed by the platinum-silica interface. This tandem catalysis was propanal. The cubic shaped nanocrystal layers can be used to build metal–metal oxide interfaces with large contact areas.
Yang says that tandem catalysis will help multiple sequential reactions in applications are essential to produce chemicals in a selective manner, such as in artificial photosynthesis that involves multiple chemical responses sequentially including water reduction and oxidation, and carbon dioxide reduction.
This research was funded by the DOE Office of Science.