The chemistry professor of Iowa State University and Ames Laboratory scientist, Klaus Schmidt-Rohr and his team used nuclear magnetic resonance (NMR) spectroscopy to study bone structure, whose stiffness is influenced by tiny nanocrystals of carbonated apatite.
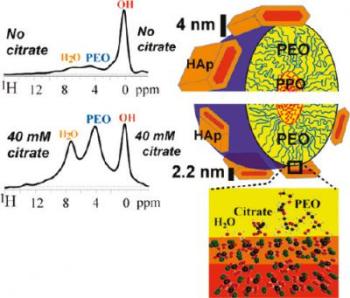 The effect of citrate concentration on the size of hydroxyapatite crystals fabricated with self-assembling block copolymer templates
The effect of citrate concentration on the size of hydroxyapatite crystals fabricated with self-assembling block copolymer templates
The tiny nanocrystals of the bone structure consist of calcium phosphate, which is present in an organic matrix comprising of a fibrous protein, collagen.
By comprehending the nanostructure of natural materials, researchers could design lightweight and durable materials with low energy consumption to obtain energy-saving products.
Klaus Schmidt-Rohr, stated that the bones are hard due to the presence of the organic collagen matrix and stiff because of the inorganic apatite nanocrystals. The nanocrystals with minute thickness of 3 nm offer mechanical properties to inhibit crack propagation.
Researchers were not able to figure out the formation of apatite nanocrystals and why they did not become thicker. Yanyan Hu, a graduate research assistant, succeeded in removing citrate from cow bone and replacing it with carbon 13 (C13)-enriched citrate.
This replacement enabled the bone sample’s NMR signals to increase 30-fold. An exact matching of peaks was observed affirming the occurrence of citrate in areas of apatite nanocrystals formation. Schmidt-Rohr added that it was challenging to fit citrate into the apatite crystal lattice. For the above reason, citrate must be attached to the surface of the nanocrystals for size stabilization to prevent further growth.
The research team also found that when citrate is present in high concentrations, the apatite nanocrystals in bone become thin. This observation was further proved when a polymer template was used with different concentrations of citrate to produce apatite nanocrystals. At higher concentrations, the nanocrystals were thinner and offered more resistance to crack propagation.