Scientists have found basic steps to charge water droplets of nanoscale dimensions and explained the hydrogen emission mechanism from irradiated water.
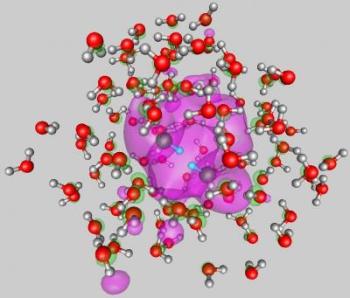 The internal attachment mode of two electrons to a water cluster comprised of 105 molecules
The internal attachment mode of two electrons to a water cluster comprised of 105 molecules
When the number of molecules in a water cluster rises above 83, a double negatively charged nano droplet is formed. This is because two excess electrons get attached to it forming dielectrons. The scientists also established with theoretical and experimental proof that in droplets containing 105 molecules or above, the excess dielectrons take part in a water-splitting mechanism to liberate molecular hydrogen and form two solvated hydroxide anions.
The Director of the Center for Computational Materials Science (CCMS) at Georgia Tech, Uzi Landman, stated that the process of attaching multiple electrons to water droplets is managed by balancing the forces that attach the electrons to the molecules of polar water and the strong repulsive force amongst the negatively charged electrons.
He added that the when an electron attaches itself to the cluster, it affects the equilibrium between the water molecules that are hydrogen-bonded and this equilibrium can be counterbalanced with the attractive forces. To measure the strength and pattern of single electron and two-electron charging of nano-scale water droplets, they utilized quantum mechanical molecular dynamics simulations that have never been previously used in this field.
Studies on clusters of controlled size enable scientists to explore intrinsic characteristics of finite-sized material aggregates. They can also investigate about the size-dependent growth of materials properties from the molecular nanoscale level to the condensed phase.
Researchers showed the basic dielectron charging processes of water droplets of microscopic dimensions and offered detailed explanation of water-splitting reaction owing to double charging with the use of large-scale, advanced first-principles dynamic simulations and time-of-flight high-resolution mass spectrometer at the CCMS. All valence and excess electrons were subjected to quantum mechanical treatment.
The mass spectrometric measurements showed that individually charged clusters consisted from six to hundreds of water molecules. However, for clusters containing more than 83 molecules, two excess electrons were found for the first time. And for clusters with 105 water molecules or more, the mass spectra gave direct proof for the loss of a single hydrogen molecule.