MIT researchers developed carbon nanotubes that are capable of accumulating solar energy for consumption whenever required.
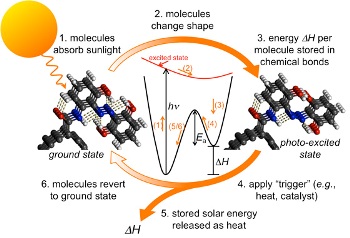
Storing solar energy in chemical form offers unique benefits, as the chemical material will not lose any of its stored energy for a long time period. But, such chemicals required for performing storage and conversion either decomposed in a few cycles or used the ruthenium element, which is costly and rare.
MIT researchers discovered precisely how fulvalene diruthenium, which is useful for storing solar energy reversibly, as it does not decompose, performed storage successfully. MIT associate professor Jeffrey Grossman and postdoc Alexie Kolpak stated that by having a good knowledge of this process, it is possible to easily identify compounds made from low-cost and abundant materials. The results of their study have been recorded online in Nano Letters.
Grossman and Kolpak produced a new material by combining carbon nanotubes and azobenzene. Grossman says that the molecules developed with nanoscale templates to render shape and restrict their physical design, features unique properties that are not present in the individual materials. Kolpak says that the new chemical system is cheaper than the previous compound of ruthenium and offers high-efficiency in storing power in limited spaces. Kolpak claims that the energy density of the new chemical system is the same as lithium-ion batteries. The new approach can be used to produce solar energy, says Grossman. He added that this technique integrates storage and energy harvesting into one step.
One disadvantage of this approach is that generation of electricity would involve another conversion step with the help of thermoelectric equipment or by generating steam to power a generator. This innovative technique can be used for developing photoactive molecules for solar storage and solar thermal fuels.