In a paper, ‘Anomalous Nuclear Quantum Effects in Ice,’ reported in the Physical Review Letters journal, a team of scientists from the Stony Brook University Department of Physics & Astronomy and the Department of Condensed Matter Physics at Universidad Autónoma de Madrid (UAM) has for the first time explained about a mystifying water anomaly in ice.
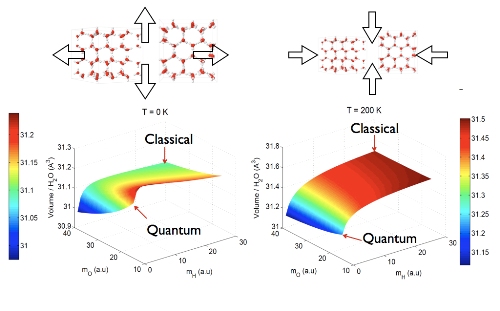 The figure shows the hexagonal ice crystal and how quantum mechanics modifies the structure with respect to a purely classical crystal. At T=0 the classical crystal is larger than the quantum crystal, but at high temperature (T=200 K) the opposite occurs and the quantum crystal shrinks. This is contrary to any other material, where the quantum crystal always expands, at all temperatures.
The figure shows the hexagonal ice crystal and how quantum mechanics modifies the structure with respect to a purely classical crystal. At T=0 the classical crystal is larger than the quantum crystal, but at high temperature (T=200 K) the opposite occurs and the quantum crystal shrinks. This is contrary to any other material, where the quantum crystal always expands, at all temperatures.
Marivi Fernandez-Serra, one of the researchers, stated that this rare property of water ice has to be included in the water anomaly list as an illustration wherein quantum effects are uncharacteristic and rise with temperature.
In this study, the scientists demonstrated that the volume of water ice relies on hydrogen and oxygen atoms’ quantum ‘zero-point’ motion in a way opposite to normal materials. For instance, crystals starts shrinking when cooled. However, due to ‘zero-point’ motion, shrinking of crystals gets stopped when the temperature approaches absolute zero. This behavior is caused by the Heisenberg Uncertainty Principle, which explains that two physical properties cannot be measured, controlled or identified simultaneously on the same accuracy level.
In crystals, quantum effects turn out to be less significant at high temperatures, thus the differences in volume lower with temperature, said Fernandez-Serra. On the other hand, volume difference in D20 or heavy water rises with temperature, which is surprising.
Based on the findings the research team proposed theoretical model, which is supported by computational simulation and an X-ray diffraction test conducted at Brookhaven National Laboratory’s National Synchrotron Light Source. According to the theoretical model, the effect is a result of the unique characteristic of the hydrogen bond.