Thomas Edison developed the rechargeable nickel-iron battery in the early 1900s. Known for its durability and reliability, the battery was used as a back-up source for power requirements of the mines and railroad industries.
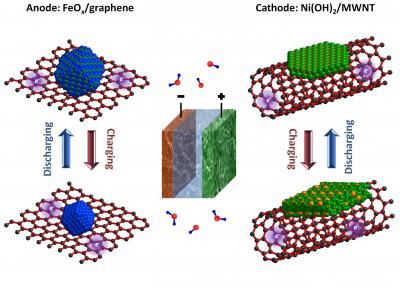 Stanford's ultrafast version of Edison Battery (Hialiang Wang, Stanford Universit)
Stanford's ultrafast version of Edison Battery (Hialiang Wang, Stanford Universit)
Until the 1920s, electric cars were also powered using nickel-iron batteries. However, there were limitations associated with the battery such as very long charging time and slow rate of discharge. While the batteries served as an economical and non-toxic replacement for corrosive lead-acid batteries in use at that time, their drawbacks led to a decline in usage. Currently they are used only to store excess power generated by wind turbines and solar panels.
Chemistry professor, Hongjie Dai and his colleagues at Stanford have given a makeover for the Edison battery and have achieved drastic improvement in charging and discharging rates by almost 1000 times. Conventional design comprises nickel cathode and iron anode, both mixed with carbon to enhance conductivity and bathed in alkaline solution. Dai’s team replaced this set up with iron oxide nanocrystals grown on graphene to serve as anode and nickel hydroxide nanocrystals grown on 10 concentric carbon nanotubes to serve as cathode. The new technique resulted in strengthening of the chemical bond between the carbon and metal which subsequently led to drastic improvement in performance. The arrangement facilitated the quick movement of electrons between the electrodes and outer circuit resulting in charging time of 2 minutes and discharge time of under 30 seconds. The prototype developed by Dai’s team has an output of only 1 V.
The team’s mission is to scale up the design to a bigger battery that can be employed in transportation or power grids. The team expects their battery to complement lithium-ion batteries used in electric cars as they do not possess sufficient energy density to operate on their own. The only significant drawback of the prototype is lack of charge-discharge cycling stability.