Researchers at Rice University have observed the formation of graphene in chemical vapor deposition (CVD) technique at the atomic level. The study findings have been published in the Proceedings of the National Academy of Sciences journal. Co-author, Boris Yakobson informed that electric current traverses through a pure graphene sheet with virtually no resistance, a quality that makes the nanomaterial a material of choice for electronic applications such as touchscreens.
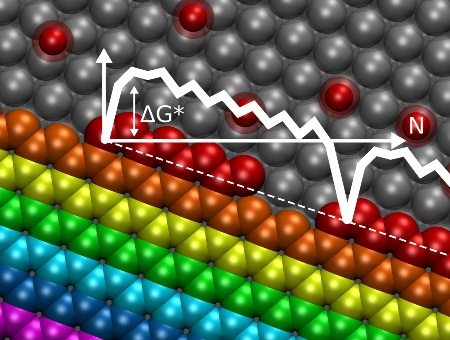 Rice University researchers have come up with a set of calculations to predict how graphene grows in the process known as chemical vapor deposition. The graph set against an illustration of graphene growing on a nickel catalyst shows the initial energy barrier a carbon atom must overcome to join the bloom; subsequent atoms face an ever-smaller energy barrier until the process begins again for the next line. (credit: Vasilii Artyukhov/Rice University)
Rice University researchers have come up with a set of calculations to predict how graphene grows in the process known as chemical vapor deposition. The graph set against an illustration of graphene growing on a nickel catalyst shows the initial energy barrier a carbon atom must overcome to join the bloom; subsequent atoms face an ever-smaller energy barrier until the process begins again for the next line. (credit: Vasilii Artyukhov/Rice University)
In CVD, the exposure of a heated carbon source to a metal catalyst takes place to grow graphene. The researchers measured the energies of individual atoms as they accumulate to create graphene at the ‘nanoreactor.’ They used their expertise in crystal growth for their nanoreactor theory. They discovered that some graphene patterns are more likely to appear than others at equilibrium based on the catalyst utilized.
Yakobson explained that the edges and boundaries of graphene decide the sheet’s overall mechanical, electrical and magnetic properties. Hence, understanding the conditions that determine the edges like armchairs or zigzags or skewed edges is significant to scientists attempting to grow the nanomaterial for making electronic components.
The researchers proposed a comprehensive model describing the migration of atoms from the feedstock, normally a carbon-rich mist in a CVD furnace, to the catalyst and eventually to the graphene lattice. The final shape of graphene relies on the delicate interaction of energies and growth rate. Like water, atoms always follow the least resistant path, but slight temperature variations and changes in the carbon vapor density can change this path.
Using density functional theory, the researchers calculated graphene growth for all possible edge orientations under different catalysts such as cobalt, copper, iron and nickel. They discovered the possibility of mapping the energy levels of atoms when they exit the vapor and reach the lattice at the nanoreactor.
The researchers found that the shape of the edge pattern is decided by how efficiently the energy is used. High energy is needed to form a new row for zigzag edges; however, subsequent row’s atoms align easily and rapidly. The initial energy barrier for armchairs is smaller and remains the same for additional atoms that dock. Skewed edges demonstrate the highest growth rate due to their smallest energy barrier. Moreover, carbon vapor with atom pairs dubbed dimers may aid in faster and superior-quality graphene growth. In addition, the lagging zigzag edges are a barrier that helps dictate the overall shape of the graphene sheet irrespective of the metal substrate. Other kinetic factors also lead to variations that form flowers, stars or asymmetric shapes.
The researchers also found that open-pentagon armchair edges are the preferred growth pattern on nickel, cobalt and iron catalysts at equilibrium, while zigzag edges are the most likely growth pattern on a copper catalyst. They also discovered a mathematical proof that some defects, wherein five- and seven-atom polygon pairs substitute adjacent hexagons, are improbable to appear other than a vacuum, an unlikely scenario for graphene growth.