Iron oxide is one of the most commonly occurring mineral in the soil that is capable of modifying the conditions of surrounding water and soil.
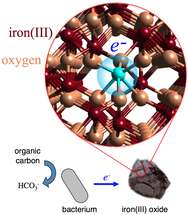 The reduction of iron(III) oxide minerals (Image courtesy Benjamin Gilbert, Lawrence Berkeley National Laboratory
The reduction of iron(III) oxide minerals (Image courtesy Benjamin Gilbert, Lawrence Berkeley National Laboratory
The important chemical changes in the naturally occurring iron are the result of electrons hopping between iron and other minerals, biological agents or water. An electron transferring to the surface of iron (III) oxide results in the creation of iron (II) sites in the mineral. There are not any fixed locations for the iron (II) sites as the electrons can transfer to any site in the mineral. Water solubility of iron (II) is greater than iron (III). Hence electron hopping alters water chemistry and soil mineralogy owing to the solubility of iron (II).
A team of scientists from Argonne National Laboratory, Berkeley National Laboratory, Pacific Northwest National Laboratory, the Technical University of Denmark and the Polish Academy of Sciences has observed for the first time, the phenomenon of electron hopping through rust nanoparticles.
The phenomenon of electron transfer was rendered viewable only due to recent developments in photonics. The journey of the electron through the iron oxide which lasts between pico and nano seconds was captured in a series of rapid succession of images by employing extremely short pulses of X-rays. The scientists could not use electric charge to initiate the process since iron oxide is a semiconductor. The team employed a pump-probe method commonly used for photovoltaic nanomaterials. A dye molecule on the surface of the mineral was excited using laser. This resulted in the injection of an electron into the iron. The scientists established via optical transient absorption spectroscopy at the femtosecond level that light initiated electron hopping was quicker than thermally induced hopping. The same light induced technique can be used to study charge collection in solar energy devices made of metal oxides.