Nov 11 2013
Panacea Pharmaceuticals, Inc. presents three papers regarding its Nanoparticle-Based Therapeutic Cancer Vaccine at the Society for Immunotherapy of Cancer Annual Meeting at National Harbor, MD held November 8-10, 2013.
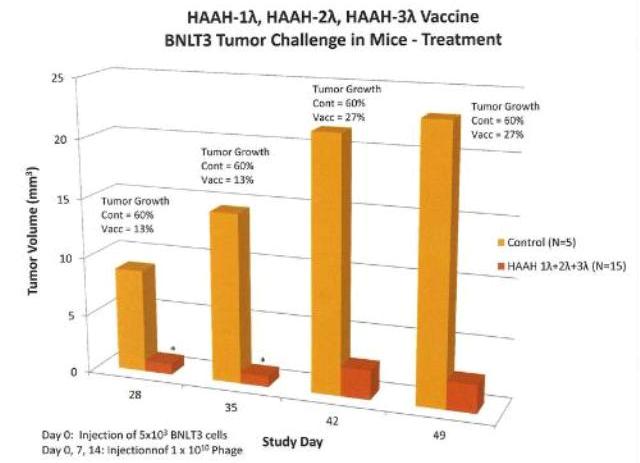 Efficacy of vaccine in mouse tumor model. Groups of mice were challenged subcutaneously on Day 0 with 5 x 103 BNLT3 tumor cells and immunized on Days 0, 7 and 14 with HAAH-1Lambda, HAAH-2Lambda, HAAH-3Lambda phage vaccines or buffer control. The graph shows growth for the control and pooled data for the HAAH constructs. Tumor growth in the nanoparticle-vaccinated animals was inhibited by nearly 90%. (PRNewsFoto/Panacea Pharmaceuticals, Inc.)
Efficacy of vaccine in mouse tumor model. Groups of mice were challenged subcutaneously on Day 0 with 5 x 103 BNLT3 tumor cells and immunized on Days 0, 7 and 14 with HAAH-1Lambda, HAAH-2Lambda, HAAH-3Lambda phage vaccines or buffer control. The graph shows growth for the control and pooled data for the HAAH constructs. Tumor growth in the nanoparticle-vaccinated animals was inhibited by nearly 90%. (PRNewsFoto/Panacea Pharmaceuticals, Inc.)
The three papers are titled:
Design, Development and Production of Nano-Particle-Based Anticancer Vaccine Targeting Human Aspartyl (Asparaginyl) β-Hydroxylase (HAAH)
Immunogenicity of a Lambda Phage-Based Anti-Cancer Vaccine Targeting HAAH
Inhibition of Tumor Growth in vivo by a Nanoparticle-Based Therapeutic Cancer Vaccine Targeting HAAH
Panacea has chosen to target the HAAH molecule for its proprietary therapeutic cancer vaccine because HAAH is expressed on cancer cells and not on non-cancer cells and its function is consistent with the etiology of cancer, i.e., is associated with growth, motility and invasiveness of cancer cells. Monoclonal antibodies to HAAH have proven to have efficacy in both in vitro and in vivo tumor models. HAAH is, however, an embryonic antigen, and as such, presents self-antigen tolerance. The present vaccine entity is designed to overcome this tolerance by altering the presentation of the antigen and by providing an immunostimulant. The nanoparticle is neutralized bacteriophage construct containing portions of HAAH, is easy to produce and is generally regarded as safe. The nanoparticle vaccine carries 200-300 copies of HAAH fragments of approximately 25 kDa molecular weight on its surface. The nanoparticle vaccine also contains DNA fragments that present the phage CpG motif to activate the MHC class II pathway. We have engineered and manufactured the nanoparticle vaccine to meet FDA requirements for human use.
The nanoparticle therapeutic cancer vaccine targeting HAAH is clearly immunogenic in mice despite the high degree of homology to the human protein that makes the AAH resemble a self-antigen. All three HAAH-lambda constructs were immunogenic and the antibody response was dose-dependent. The nanoparticle therapeutic cancer vaccine targeting HAAH is effective in inhibition of tumor growth in mice under various conditions. Each of the three constructs can inhibit tumor growth. The vaccine can slow the growth of tumors prophylactically or by treatment simultaneous with or subsequent to establishment of solid tumors.
"We are excited to report that the nanoparticle-based therapeutic cancer vaccine targeting HAAH can be manufactured readily and is effective in tumor models", said Hossein Ghanbari, Ph.D., CEO and CSO of Panacea. "The HAAH phage constructs are now being developed as clinical candidate vaccines and we are taking all the steps to prepare for an IND submission to FDA", said Steven Fuller, Ph.D., COO of Panacea.