Sep 3 2014
Biological aggregation is a critical, yet often overlooked factor in the medical application of nanoparticles. Here we systematically characterize the effects of aggregation on both radiofrequency heating and magnetic resonance image (MRI) contrast of magnetic iron oxide nanoparticles (IONPs), including detailed analysis of the aggregate morphologies based on quasi-fractal descriptions.
While aggregation is shown to produce significant reductions in both heating and MRI contrast, we also present a new method to quantify and correlate these effects for clinical applications, such as cancer hyperthermia, utilizing sweep imaging with Fourier transform (SWIFT) MRI. Therefore, the results of this work not only present new methods to systematically examine a poorly understood area of nanomedicine (not unique to magnetic nanoparticles), but also demonstrate an imaging platform to account for it in the clinic.
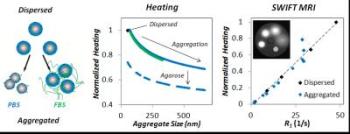 Nanoparticle aggregation in biological systems can occur due to interactions with ions (phosphate buffered saline, PBS), proteins (fetal bovine serum, FBS), extracellular matrix, and cells. Aggregation of iron oxide nanoparticles leads to significant reductions in heating potential and MR imaging contrast, but here we also demonstrate an empirical correlation between the two, providing new ability for image guidance of iron oxide nanoparticle heating in the clinic. Credit: Authors
Nanoparticle aggregation in biological systems can occur due to interactions with ions (phosphate buffered saline, PBS), proteins (fetal bovine serum, FBS), extracellular matrix, and cells. Aggregation of iron oxide nanoparticles leads to significant reductions in heating potential and MR imaging contrast, but here we also demonstrate an empirical correlation between the two, providing new ability for image guidance of iron oxide nanoparticle heating in the clinic. Credit: Authors
Jack Hoopes, Ph.D., D.V.M., of Dartmouth College and Principal Investigator at the Dartmouth Center for Cancer Nanotechnology Excellence (DCCNE) says, "It is now clear that development of a clinically effective magnetic nanoparticle cancer treatment will require nanoparticles that have significant magnetic field-initiated heating potential and the ability to be observed accurately in a non-invasive, real-time manner. Your University of Minnesota MR-SWIFT imaging technology is the first to demonstrate that magnetic nanoparticles can be observed and quantified, with excellent contrast and resolution, at therapeutically meaningful levels using a conventional imaging technology. Equally important is your demonstration that SWIFT MRI can be used to predict the level of therapeutic heating for a specific amount of tumor iron (IONPs) and that IONP aggregation, in the tumor, is critical to understanding heat generation. Our DCCNE looks forward to working with you and your recently developed techniques to enhance our upcoming IONP clinical trials." The DCCNE is attempting to conduct the first in-human clinical trials related to IONP based cancer hyperthermia in the U.S.
On-going work at the University of Minnesota and Dartmouth College plans to expand on the preliminary in vivo studies presented here and demonstrate intra-operative concentration and SAR mapping for IONP treatments that will translate to the clinical setting. This includes opportunities to increase the range of measurable IONP concentrations beyond those already established (up to 3-4 mg Fe/ml) through increased acquisition bandwidth or multi-band-SWIFT. This not only has im-portant implications for cancer hyperthermia applications, but may provide a platform to perform transient, non-invasive biodistribution studies on IONPs. Finally, as the interparticle interactions due to aggregation were determined to be detrimental to the IONPs' heating potential, the authors are also investigating new ways to account for it using SWIFT (as shown here) and controlling it through new coatings which additionally increase the potential for future targeting and drug payload inclusion.