Oct 1 2014
Silver nanoparticles are well known for their anti-bacteria properties[1-4]. One of the main routes by which they may act as an anti-bacteria agent, is through attaching themselves to the thiol group present on the cellular membrane surface and hence disrupting the membrane's function[5].
Hence, it is crucial to gain a greater understanding of this complex silver-thiol interaction to determine silver nanoparticles' role in biological systems. With thiols, silver nanoparticles have been proposed to form various types of compounds with different structures[6-8]. One of the plausible reaction routes suggested for organothiols is[6,7]: 4 RSH + 4 Ag + O2 4 AgSR + 2 H2O (1)
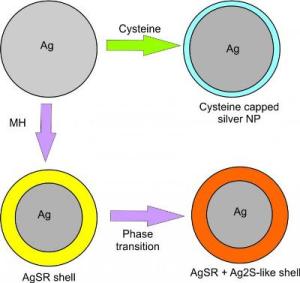 This figure shows the interaction between silver nanoparticles and the two thiols of 6-mercaptohexanol (MH) and cysteine. Credit: ©Science China Press
This figure shows the interaction between silver nanoparticles and the two thiols of 6-mercaptohexanol (MH) and cysteine. Credit: ©Science China Press
It has been shown through scanning electron microscopy that AgSR remained on the surface, forming a shell[6]. Battocchio et al. suggested a shell of more than one species of silver thiolates – a mix of AgSR and Ag2S-like complexes were formed when aromatic organothiols were added during silver nanoparticle synthesis[8]. From the above, it known that the interaction between silver nanoparticles and thiol is complicated. Therefore, in this study, we presented a comparative view of silver-thiol interaction for MH and cysteine through voltammetric and spectroscopy experiments.
Cyclic voltammograms were performed in the presence of MH or cysteine to ascertain the changes in the ease and extent of oxidation of silver nanoparticles to silver(I) ions. The UV-vis spectra of silver nanoparticles were recorded to examine the changes in the nanoparticles' surface properties in the presence of thiols. From the experimental data, it was concluded that MH interacts with silver nanoparticles to give a sparingly soluble silver(I) thiolate complex AgSRm (Rm = -(CH2)6OH) on the surface. It was also inferred that the AgSRm complex on the nanoparticle surface undergoes a phase transition to give a mixture of AgSRm and Ag2S-like complexes due to the presence of two signals in both electrochemical voltammograms and UV-vis spectrums[8]. In contrast, when silver nanoparticles were exposed to cysteine, the citrate capping agent on the silver nanoparticles was replaced by cysteine to give cysteine capped nanoparticles. As cysteine capped nanoparticles form, the electrochemical data displayed a decrease in oxidative peak charge but the UV-vis spectra showed a constant signal. Therefore, cysteine capped nanoparticles were suggested to have either inactivated the silver surface or else promoted detachment from the electrode surface. Figure 1 shows schematically the route which silver nanoparticles interact with MH and cysteine.
It may be concluded that no general mechanism for the interactions of thiol with silver nanoparticles exist and that each thiol must be treated individually.
References
[1]R. K. Kunkalekar, M. M. Naik, S. K. Dubey, A. V. Salker, J. Chem. Technol. Biotechnol. 2013, 88, 873-877.
[2]Y. Park, H. J. Noh, L. Han, H.-S. Kim, Y.-J. Kim, J. S. Choi, C.-K. Kim, Y. S. Kim, S. Cho, J. Nanosci. Nanotechnol. 2012, 12, 7087-7095.
[3]M. Sureshkumar, D. Y. Siswanto, C.-K. Lee, J. Mater. Chem. 2010, 20, 6948.
[4]C. Marambio-Jones, E. M. V. Hoek, J.Nanopart. Res. 2010, 12, 1531-1551.
[5]A. Lapresta-Fernández, A. Fernández, J. Blasco, TrAC, 2012, 32, 40-59.
[6]S. M. Ansar, G. S. Perera, P. Gomez, G. Salomon, E. S. Vasquez, I. W. Chu, S. Zou, C. U. Pittman, K. B. Walters, D. Zhang, J. Phys. Chem. C 2013, 117, 27146-27154.
[7]A. Andrieux-Ledier, B. Tremblay, A. Courty, Langmuir 2013, 29, 13140-13145.
[8]C. Battocchio, C. Meneghini, I. Fratoddi, I. Venditti, M. V. Russo, G. Aquilanti, C. Maurizio, F. Bondino, R. Matassa, M. Rossi, S. Mobilio, G. Polzonetti, J. Phys. Chem. C 2012, 116, 19571-19578.