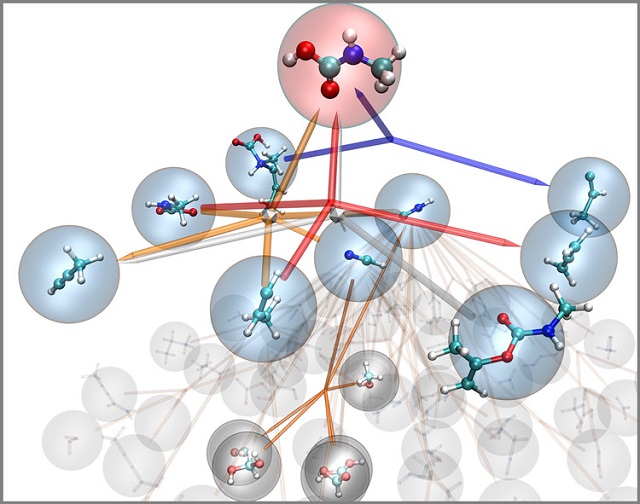
 The nanoreactor works like a virtual chemistry set to discover new reactions and mechanisms. This diagram describes the reaction network for methylcarbamic acid, identifying all the reactions involving it or leading to its production. Courtesy Todd Martinez
The nanoreactor works like a virtual chemistry set to discover new reactions and mechanisms. This diagram describes the reaction network for methylcarbamic acid, identifying all the reactions involving it or leading to its production. Courtesy Todd Martinez
Chemists from Stanford University have developed a computer model that could provide details on the chemical reactions taking place during the Urey-Miller experiment, but also determine the possible products of the experiments. The model has been termed as the nanoreactor and enables scientists to determine mechanisms or reactions revealing the possibilities of new drug formation or improving the battery or fuel combustion efficiency.
The model described in Nature Chemistry journal works by manually entering the target chemical structure, followed by setting the environmental parameters like pressure or temperature. The quantum mechanical problems of each electron in the interacting molecule can be solved using the algorithms while recording each step simultaneously.
Unlike the complex traditional approach of manually recording the electron movements, the nanoreactor simulates 100 to 200 atoms at a time producing desired results in a short span of time. As a result, the nanoreactor could find applications in refining critical chemical processes such as combustion reaction used in gas-fueled automobiles.
In general, combustion involves several reactions which can be revealed using this concept thereby identifying a suitable reaction for sustainable combustion. This also helps in mitigating soot formation or avoiding other undesirable reaction mechanisms.
The chemists are now working to develop catalysts that enhance reaction efficiency in fuel cell applications, using the nanoreactor. In addition, they expect the nanoreactor to provide an insight of the biochemical reactions in the human body, paving the way for the development of new drugs.
You can just hit a button and it will tell you all the reactions that are important It uses a hybrid approach that incorporates physics and machine learning to discover all the possible ways that your chemicals might react, and that might include reactions or mechanisms we've never seen before.
Senior author of the research, Todd Martinez
References