Nov 27 2014
Researchers at the University of Kansas (KU) have fabricated a new material using single atomic layers of graphene and tungsten disulfide.
Similar to a Legos assembly, two different atomic sheets were made to interlock using a nanofabrication technique. This innovative material holds potential for applications in flexible electronics and solar cells.
 Lego physics
Lego physics
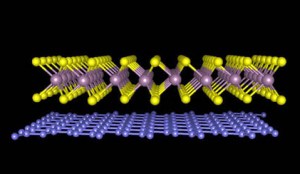
Combining different atomically thin materials has historically been a difficult task. Most materials have different atomic arrangements which do not allow them to connect together at the interface. This is similar to playing with Legos from different manufacturers and different sizes. Hence only materials that have very similar atomic arrangements and properties can be used to make new materials.
KU researchers used a layer-by-layer nanofabrication technique to build the new material. Atoms in conventional materials are bound together strongly in all directions. The atoms in atomically thin layers of the new materials are strongly bound with their neighboring atoms.
However, these atomic sheets are linked together by weak van der Waals forces.
Due to this weak interlayer connection, different types of atomic sheets can be used and they can be placed on top of each other.
This is similar to using Legos having flat bottoms. This innovative method enables production of numerous new materials that collectively exhibit the properties of each individual material used.
The research team chose tungsten disulfide and carbon for producing efficient solar cell materials.
Tungsten disulfide atoms can efficiently absorb sunlight and convert it into electricity, while the atoms in graphene can efficiently move electrons. The combination of these two materials resulted in a new material with the properties of both tungsten disulfide and graphene.
A single layer of tungsten disulfide atoms was lifted using scotch tape and applied on a silicon substrate. Following this, a single layer of carbon atoms was lifted from a graphite crystal.
The graphene layer was placed over the tungsten disulfide layer using a microscope. This material was then heated for half an hour at 500°F, in order to remove any glue between the material layers.
At the Ultrafast Laser Lab, the tungsten disulfide layer was excited using a laser pulse, causing shifting of the electrons that absorbed laser pulse energy to graphene within one picosecond. This means that the new material has the advantageous properties of graphene and tungsten disulfide.
This study has been published in the Nature Communications journal.