Mar 18 2015
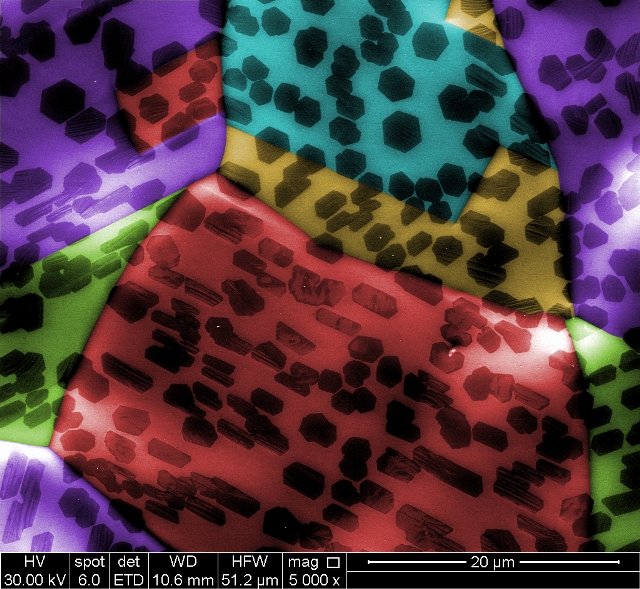 Graphene islands formed in two distinctly different shapes on separate grains of copper (colored in blue and red) grown simultaneously because the substrates' atomic lattices have different orientations, according to Rice University researchers. Image by Yufeng Hao/coloring by Vasilii Artyukhov
Graphene islands formed in two distinctly different shapes on separate grains of copper (colored in blue and red) grown simultaneously because the substrates' atomic lattices have different orientations, according to Rice University researchers. Image by Yufeng Hao/coloring by Vasilii Artyukhov
Scientists at Rice University have discovered that the geometric relationship that exists between graphene and the substrate determines the manner in which the shapes of the islands emerge.
The team studied patterns of graphene that were grown using chemical vapor deposition method in a furnace.
Substrates are the underlying material, and carbon assembles over this material atom by atom. The researchers showed the manner in which the crystalline arrangement of atoms in substrates controlled the way in which the islands formed.
Nickel and copper are commonly used substrates for graphene growth. Boris Yakobson, a theoretical physicist at Rice University, had performed this study along with Vasilii Artyukhov, a postdoctoral researcher.
“Experiments that show graphene’s amazing electronic properties are typically done on mechanically exfoliated graphene,” Artyukhov said. “That limits you in terms of the flake size, and it’s expensive if you need a lot of material. So everybody’s trying to come up with a better way to grow it from gases like methane (the source of carbon atoms) using different substrate metals. The problem is, the resulting crystals look different from substrate to substrate, even though it’s all graphene.”
Yakobson said researchers often see odd-shaped graphene islands grown by chemical vapor deposition, “and we have all wondered why. In general, this is very surprising, because in graphene, the six sides should be identical.”
Other shapes including triangles are formed due to symmetry breaking. This takes place when systems that usually produce regular shapes take a break and form shapes that are less regular.
When carbon atoms that are floating in the hot fog in a chemical vapor deposition furnace settle down on the metallic substrate, graphene is formed. The atoms initially link up in the form of the usual characteristic six-sided rings.
However, when the island grows the shape takes on different forms that include triangles, hexagons, elongated hexagons and other random structures. A strong correlation was found to exist between the island’s final shape and the atomic arrangement on the substrate’s exposed surface, which could be in various shapes including square, rectangular, triangular or other shapes.
The road map that the substrate set out was followed by individual atoms. This was shown by a microscope image having two grains of copper substrate that were hosting two different shapes of graphene.
This occurred even though the conditions for growth were the same. All of the graphene islands on one grain were nearly perfect hexagons, while on the other grain the hexagonal islands were found to be aligned and elongated.
“The image shows the basic growth mechanisms are the same, but the difference in the islands is due to the subtle differences between the crystallographic surfaces of the graphene and copper,” Yakobson said.
The edges of graphene play a very important role in its electronic properties. Hence, it is vital to know how it grows. The manner in which single atoms fall into an equilibrium state when they balance the energies between the substrate’s atoms and the neighboring carbon atoms decides whether the edge of graphene would end up in the form of an armchair, or zigzag or an in between form.
In metals, the atoms form a crystal lattice or a specific arrangement. It could be a pure copper lattice, which is known as a 'face-centered cubic'. However, in copper foils that are polycrystalline materials the individual grains can possess different surfaces. Copper foils are commonly used as substrates for graphene-growth.
“Depending on the way you cut a cube in half, you can end up with square, rectangular or even triangular faces,” Artyukhov said. “The surface of copper foil can have different textures in different places. Electron microscopy showed that all graphene islands growing on the same copper grain tend to have a similar shape, for instance, all perfect hexagons, or all elongated.”
Artyukhov stated that the symmetry of the grains’ surfaces is inherited by the islands and hence grow rapidly in certain directions. This answers the question of peculiar distribution of shapes.
The islands merge to form a larger graphene film when the growth process continues for a sufficiently long period. The atoms seek out equilibrium in specific places where the alignment of carbon lattices does not take place.
Here the atoms form grain boundaries and in turn control the electronic properties of the larger graphene sheet’s electronic properties. It is widely desired to find out ways to control the boundaries of graphene to control its semiconducting properties.
“A good understanding of this process gives directions on how to organize the mutual orientation of islands,” Yakobson said. “So when they fuse you can, by design, create particular grain boundaries with particularly interesting properties. So this research, more than just satisfying our curiosity, I s very useful.”
Yakobson suggests that the calculations used in this study could also be used for the growth of hexagonal boron-nitride, molybdenum disulfide or other such 2D materials. Researchers are conducting studies on these materials due to their potential for applications in electronics.
Rodney Ruoff, director of the Center for Multidimensional Carbon Materials at the Ulsan National Institute of Science and Technology, Ulsan, South Korea; and Yufeng Hao, a research scientist at Columbia University are the co-authors in this study.
This study has been published in the journal, Physical Review Letters.