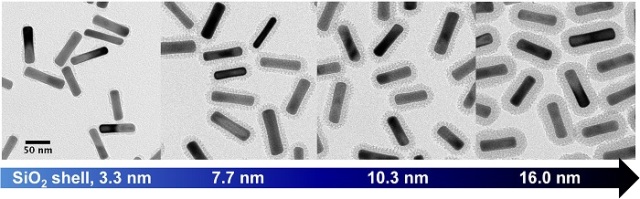
 Gold nanorods with silica shells of varying thicknesses. (Image credit: Joe Tracy)
Gold nanorods with silica shells of varying thicknesses. (Image credit: Joe Tracy)
NC State University researchers have perfected a method of coating gold nanorods using silica shells. This technique is capable of producing nanorods on a large scale without imparting remarkable control over the shell thickness.
Gold nanorods are being studied for a large number of biomedical applications. The new research helps in creating highly stable gold nanorods and also in functionalizing the shell surface chemically.
Owing to their surface plasmon resonance i.e. the ability to absorb and scatter light, gold nanorods have a number of potential applications. Further, the wavelength of absorbed light can be controlled by altering the dimensions such as aspect ratio of the nanorods.
“This characteristic makes gold nanorods attractive for use in catalysis, security materials and a range of biomedical applications, such as diagnostics, imaging, and cancer therapy,” said Joe Tracy, a materials science and engineering researcher at NC State who is senior author of a recent paper on the improved technique.
Gold nanorods are ideal for photothermal heating where the absorbed light is converted into heat. Upon exposing gold nanorods to excess light, the nanorods tend to lose their shape and convert into spheres thus losing their optical characteristics.
Coating the nanorods with silica shells is one way of making them to retain their shape during photothermal heating. However, the coating allows the light to pass through the nanorods. Thin shells ensure negligible changes in the size of the nanorods, allowing arrangement of rods as dense assemblies. By contrast, thicker shells serve as buffers wherein the nanorods are prevented from being formed as bunches and protect them from their surroundings.
“The silica shells offer multiple benefits – and our modified approach to coating gold nanorods with silica shells has two distinct advantages,” Tracy said.
“First, we have demonstrated that our technique can be carried out on a large scale – up to 190 milligrams. Second, we offer improved control over shell thickness. We can consistently create uniform shells as thin as 2 nanometers,” Tracy added.
The modified technique is a two-step procedure.
“First we apply a reagent called TEOS to the gold nanorods in solution. Once in solution, the TEOS begins to form a silica shell on the nanorods. We then introduce another reagent called PEG-silane into the solution. This stops the shell from growing thicker,” says Wei-Chen Wu, a Ph.D. student in Tracy’s lab and lead author of the paper.
The research paper titled “Large-Scale Silica Overcoating of Gold Nanorods with Tunable Shell Thicknesses,” is published in the Chemistry of Materials journal. The research was supported by Engineering Center under grant DMR-1121107, the NSF’s Research Triangle Materials Research Science, the National Institutes of Health under grant 1R21HL111968-01A1 and the National Science Foundation under grant DMR-1056653.