Mar 25 2015
Dartmouth researchers and bioengineers develop novel system to improve cancer treatment
To quantify rare tumor markers that will allow oncologists to make prognoses and select therapies, John X.J. Zhang, PhD led a team of bioengineers from the Thayer School of Engineering at Dartmouth in demonstrating a novel system that couples nano-engineered particles and microfluidic chips for capturing and manipulating circulating tumor cells (CTCs). The microscale immunoassay can be further interfaced with a fluorescent microscope for cancer cell imaging. Their paper, "Microscale Magnetic Field Modulation for Enhanced Capture and Distribution of Rare Circulating Tumor Cells," was published in Scientific Reports, Nature Publishing Group.
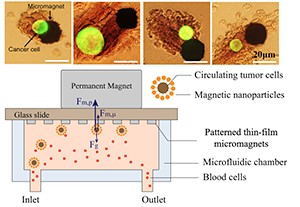 Microchips for blood screening, where cancer cells are attracted to array of micromagnets for further analyses
Microchips for blood screening, where cancer cells are attracted to array of micromagnets for further analyses
"This project demonstrates that a relatively simple blood test may eventually be able to provide unambiguous information to doctors about particular cancers in individuals," said Zhang.
Live cells represent vital model systems for studying organism development and human disease. Invasive cancers shed tumor cells into the blood and, by detecting those cells at an early stage, physicians will be able to determine a patient's prognosis and best alternatives for therapies. The capture and immunophenotyping of CTCs shed by cancers at an early stage, and postulated as the mechanism of development of recalcitrant metastatic disease, is envisioned to revolutionize risk assessment, treatment selection, response monitoring, and development of novel therapies.
Zhang's team focused on creating a new interface between living cells and hybrid microsystems, which enabled rigorous design, modeling, manufacturing, and validation of high-performance and massively deployable bio-analytical microsystems for point-of-care and globally-relevant diagnostic applications.
"The concept is to use novel cell-machine interfaces, integrated sensing, actuation and biomarker recognition functionalities to isolate these rare cells (1 per 109 hematologic cells) from whole blood to determine malignancy unambiguously," Zhang said. "We will base the quantitative assessment on multiple tumor markers."
The Dartmouth group demonstrated they can effectively combine the benefits of immunomagnetic assay with microfluidic technology for high-throughput CTC screening. This resulted in a high sensitivity assay for increased cell capture rate and reduced cell aggregation that will be suitable for down-stream analyses of CTCs at the single cell level.
Zhang's goal is to bring this technology from the bench to the clinics, enabling doctors to diagnose and manage cancer via simple blood tests. Operationalizing this technology will potentially increase the cure rate for cancers such as breast cancer.
Zhang is Professor at Dartmouth's Thayer School of Engineering. His work in cancer is facilitated by Dartmouth's Norris Cotton Cancer Center where he is a Member of the Cancer Imaging & Radiobiology Research Program.
"Microscale Magnetic Field Modulation for Enhanced Capture and Distribution of Rare Circulating Tumor Cells," was funded by the National Institutes of Health and National Cancer Institute Cancer Diagnosis Program grant 1RO1CA130070.