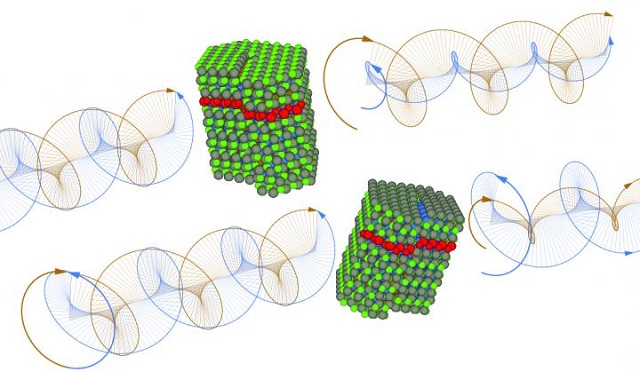
 These are levorotatory and dextrorotatory quantum dots with left and right chiral defects. Credit: Courtesy of ITMO University
These are levorotatory and dextrorotatory quantum dots with left and right chiral defects. Credit: Courtesy of ITMO University
Scientists from the Trinity College Dublin and the ITMO University have demonstrated that ordinary nanocrystals have intrinsic chirality and can be produced under normal conditions as a half-and-half mixture of mirror images of each other.
These findings have opened new possibilities in medicine, bio-technology and nanobiotechnology for applications including targeted drug delivery.
Chirality of an object is its property that allows it to be non-superimposable with its mirror image. Ever since the development of artificial nanocrystals, scientists thought that chirality was either random or completely absent in nanocrystals.
Researchers from Trinity College’s Centre for Research on Adaptive Nanostructures and Nanodevices (CRANN), partnered with collaborators from ITMO University’s Optics of Quantum Nanostructures laboratory in a joint experiment to show that standard nanocrystals were made up a 50:50 mixture of 'left' and 'right' chiral forms. Standard nanocrystals are composed of cadmium selenide quantum dots and quantum rods. Artificial chiral nanocrystals can be produced by fastening special chiral ligand molecules to the nanocrystal surface.
In the natural world, chirality is an inherent property of many objects that range from spiral galaxies to elementary particles. The human body is composed of chiral biomolecules. Many other biological objects are also composed of these chiral biomolecules. In these compounds, the 'left' form may be significantly different from the 'right' form. Among these forms, usually one form is beneficial, which could be medical benefits, while the other form, which is its antipode, would be useless. Ibuprofen is a widely used painkiller and its molecules possess two optical mirror isomers. One of these isomers is beneficial and relieves the pain, while the other is toxic and does not relieve pain.
The optical activity is considered to be an important indicator of chiral environment. It has the ability to rotate the polarized light plane to the left or right, depending upon the nanocrystal’s chiral form. Theoretically, optical activity is not observed in any normal nanocrystal solution. The absence of chirality in nanocrystals has been considered to be the cause of optical activity. In this study, the researchers have proved the opposite, by dividing the nanocrystal’s 'left' and 'right' forms.
The absence of optical activity in a solution of nanocrystals can be explained by the fact that a racemic (50:50) mixture combines 'left' and 'right' versions of nanocrystals that simultaneously rotate the plane of polarization in opposite directions, thus cancelling each other out. We explain the very existence of intrinsic chirality in nanocrystals by chiral defects that occur naturally during normal synthesis of nanocrystals.
Maria Mukhina, researcher at Optics of Quantum Nanostructures laboratory.
Yurii Gun'ko, professor at Trinity College and co-director of International Research and Education Centre for Physics of Nanostructures at ITMO University comments on potential applications of the method developed by the group:
There is a global demand for new ways of obtaining chiral nanoparticles. We believe that our method will find applications in biopharmaceutics, nanobiotechnology, nanotoxicology and biomedicine, in particular for medical diagnostics and targeted drug delivery. For example, if all commonly used nanoparticles are indeed chiral, then during interaction with a biological object 50 percent of nanoparticle mixture will penetrate into the biological object (e.g. cell), while the other 50 percent will remain outside. The implications of this conclusion are crucial for nanotoxicology area, but nobody considered them before. Another potential application has to do with the ability of chiral quantum dots to emit levorotatory and dextrorotatory polarized light, which makes it possible to create devices such as 3-D holographic displays and much more.
The scientists developed a technique for separating various forms of nanocrystals and also capture their intrinsic chirality’s manifestation. This technique could possibly be expanded and then used with various other inorganic nanomaterials.
In an unmixable two-phase solution composed of an organic solvent (chloroform) and water, nanocrystals were immersed. Nanocrystals do not dissolve in water; hence L-cysteine was added to transfer the nanocrystals in organic phase to water. L-cysteine is a chiral molecule and it is widely used for phase transfers as a ligand. Nanocrystals have hydrophobic ligands on their surface, and cysteine replaces these ligands and makes the material soluble in water.
Hence, all the nanocrystals will be in water, irrespective of the cysteine’s chiral form. When this solution was cooled and the phase transfer was interrupted at a specific point, a particular situation where the nanocrystal ensemble was equally divided between the phases that had nanocrystals – both 'left' and 'right' - in different phases.
Furthermore, removal of cysteine does not affect the nanocrystal’s optical activity due to this separation. This provides more proof to the existence of intrinsic chirality in nanocrystals.
Vladimir G. Maslov, Anatoly V. Fedorov, Alexander V. Baranov, Finn Purcell-Milton, Anna O. Orlova, and Joseph Govan were other researchers who took part in this study.
The research team has published their study titled, 'Intrinsic chirality of CdSe/ZnS quantum dots and quantum rods,' in Nano Letters.
References