Jun 26 2015
Sleeping sickness, or African trypanosomiasis, is caused by trypanosome parasites transmitted by tsetse flies and threatens millions of people in sub-Saharan Africa. The disease is considered fatal if untreated, but as it affects mostly poor people in low-income countries, treatment options are limited.
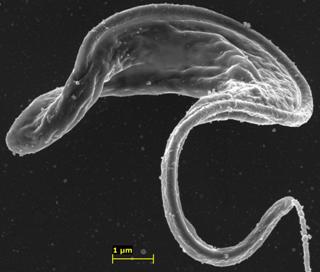 This is the parasite Trypanosoma brucei with targeted nanoparticles adhered to its surface. Credit: Garcia-Salcedo et al, CC-BY
This is the parasite Trypanosoma brucei with targeted nanoparticles adhered to its surface. Credit: Garcia-Salcedo et al, CC-BY
The existing drugs have serious side effects, and the parasites are developing resistance. A study published on June 25th in PLOS Pathogens reports a new way to circumvent drug resistance and lower the curative dose by delivering existing drugs directly into the parasite, a high-tech approach with potential applications to other infectious diseases.
Current treatment of sleeping sickness relies primarily on four drugs. Three of these drugs get into the interior of the parasite cells via the trypanosome's transport proteins that normally supply the parasite with nutrients, and drug resistance is caused by mutations that cripple these transporters. Jose Garcia-Salcedo, from the Instituto de Investigacion Biosanitaria in Granada, Spain, and colleagues reasoned that using an alternative way to get the drugs into parasite cells would circumvent resistance.
The researchers developed a drug carrier that consists of polymeric nanoparticles coated with specialized antibodies that target a small conserved (i.e., invariable) part of the parasite surface. (Much of the trypanosome surface is highly variable, which is why the chances of developing an effective vaccine have been deemed low.) They show that this new formulation reduces the minimal curative dose in a disease model, based on infections in mice, by 100-fold and, most importantly, circumvents drug resistance in a cell line that is resistant as a result of mutations in the transporter that mediates drug uptake.
The authors conclude "in summary, the development of chitosan nanoparticles loaded with current trypanocidal drugs coated by a specific nanobody against trypanosomes can reduce the minimal curative dose of these drugs, enhancing their efficacy, minimizing the toxicity and circumventing resistance mechanisms caused by mutations in surface transporters."
The implication of this proof-of-concept study of a novel technology for reversing transporter-related drug resistance, they say, "is not limited to a single nanobody used to demonstrate the technology, nor to a single drug, nor indeed to trypanosomiasis." "With a key challenge being that resistance to drugs is spreading faster than new drugs are being developed and approved", they suggest that "the use of encapsulated, nanobody-targeted drugs as described here has the potential to reverse resistance to many first-line treatments."