The National Physical Laboratory (NPL) has successfully mapped catalytic reactions at the nanoscale using tip-enhanced Raman spectroscopy (TERS).
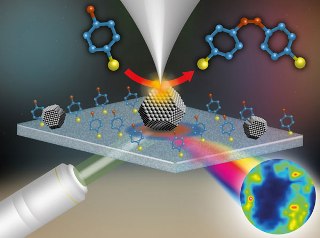 "Schematic of the TERS apparatus and the catalytic
reaction studied"
"Schematic of the TERS apparatus and the catalytic
reaction studied"
Catalysts are defined as materials that assist chemical reactions without themselves being consumed. They help in production of chemicals that could not have been produced otherwise or would have been economically not feasible for production. More than 90% of industrial chemical processes utilise catalysts, and they are used in a wide range of industries, including energy generation and pharmaceuticals. Catalysts are believed to contribute to more than 35% of the global GDP.
The industry desires chemistry that is more economical, sustainable, and greener, and this has driven the demand for new catalysts that possess better selectivity and efficiency. In this regard, identifying active sites at the surfaces where reaction takes place is important for effective design of catalyst substances that possess specific desired properties. The structure-performance relationships have to be understood. Commonly used analytical techniques do not possess the sensitivity necessary for understanding these relationships.
TERS combines the advantages of scanning probe microscopy’s nanoscale spatial resolution and surface-enhanced Raman spectroscopy’s high chemical sensitivity. These properties make TERS desirable for nanoscale characterization of surfaces, and hence, nanometre length-scale characterization of catalytic reactions.
The NPL team has successfully used TERS for nanoscale mapping of catalytic activity by finding out the catalytic nanoparticles that are on a surface. No other technique has until now matched the resolution of this reactive spectroscopic imaging technique with nanometre resolution.
The developments achieved by the team could enable widespread utilization of TERS for analyzing catalytic reactions with nanoscale resolution. Furthermore, spatial variations that have been found using this technique could aid in providing more information about reaction dynamics and molecular adsorption at surfaces. Finally, this could enable desired catalyst optimization by enabling better efficiency and control of chemical processes.
The study paper has been published in Nanoscale, a Royal Society of Chemistry journal.
References