A novel solid-state catalyst that can be used to electrolyze water, with a similar efficiency to established (and expensive) platinum catalysts, has been developed. The catalyst is graphene-based with active cobalt atoms distributed across its surface. It is hoped this research will lead to cheaper water electrolysis; which is essential for the production of hydrogen to be used in fuel cells.
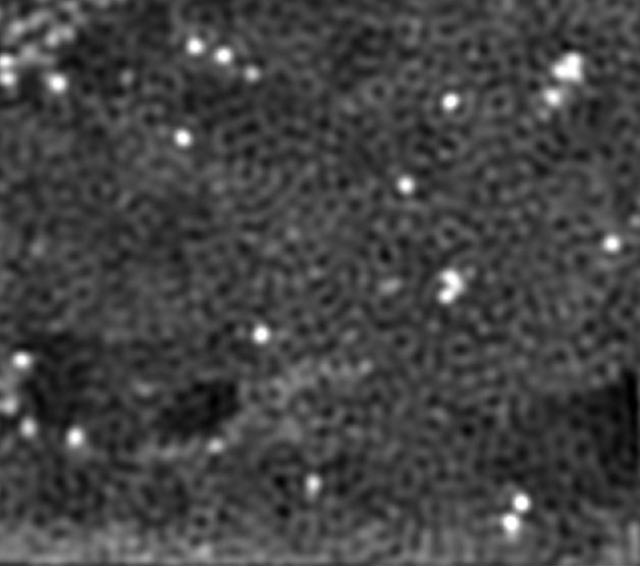 Cobalt atoms shine in an electron microscope image of a new catalyst for hydrogen production invented at Rice University. The widely separated cobalt atoms are bound to a sheet of nitrogen-doped graphene. (Credit: Tour Group/Rice University)
Cobalt atoms shine in an electron microscope image of a new catalyst for hydrogen production invented at Rice University. The widely separated cobalt atoms are bound to a sheet of nitrogen-doped graphene. (Credit: Tour Group/Rice University)
The research team claimed that doping graphene with nitrogen and then augmenting it with cobalt atoms results in a strong and effective catalyst for the electrolysis of water.
A collaborative research team from Rice lab, the University of Texas at San Antonio, the Chinese Academy of Sciences and the University of Houston developed the strong and durable catalyst. It is hoped that its discovery will eliminate the need for expensive platinum-based catalysts which are currently used for hydrogen production. Platinum catalysts lower the energy required to split water into its constituent oxygen and hydrogen atoms. This process is needed to create the hydrogen fuel that powers fuel cells.
What’s unique about this paper is that we show not the use of metal particles, not the use of metal nanoparticles, but the use of atoms. The particles doing this chemistry are as small as you can possibly get. Even nanoscale particles function at the surface level. There are so many atoms inside the nanoparticle that never do anything. But in our process the atoms driving catalysis have no metal atoms next to them. We’re getting away with very little cobalt to make a catalyst that nearly matches the best platinum catalysts.
James Tour - Rice Lab
Tour informed that during comparison analyses, the novel material almost had the same efficiency as platinum at a low onset voltages. The low onset voltage is the amount of electricity required by the catalyst to split water into oxygen and hydrogen.
The novel catalyst can be either used as a surface coating or can be changed into a material that resembles paper. Although catalysts with only one catalytically active atom have been observed in liquids this behavior is rarely achieved on a surface.
This way we can build electrodes out of it. It should be easy to integrate into devices.
James Tour - Rice Lab
The scientists found that when small quantities of cobalt salts and graphene oxide are heat treated in a gaseous environment that individual cobalt atoms are pushed into the graphene oxide sheet. Through electron microscope images, it was observed that the cobalt atoms were broadly distributed across the sheet. Next, the nitrogen-doped graphene was tested and it was found that this material does not have the required ability to start catalytic processes. However, when small amounts of cobalt salts are added the nitrogen-doped graphene was easily able to separate basic or acidic water.
(Platinum catalysts are) an extremely high-performance material. No question, they’re the best. But this is very close to them, much easier to produce and hundreds of times less expensive.
James Tour - Rice Lab
According to Tour, one-atom-thick graphene serves as a perfect substrate, thanks to its high conductivity, excellent stability and high surface area. In the new catalyst samples, it was observed that the activity reduced only slightly following 10 hours of accelerated degradation analysis.
The study has been published in Nature Communications.
The National Institute on Minority Health and Health Disparities from the National Institutes of Health; the Air Force Office of Scientific Research Multidisciplinary University Research Initiative, the National Natural Science Foundation of China, and the Welch Foundation supported the study.