Nov 5 2015
A new drug-device combination for the treatment of stroke-causing blood clots has been developed. A stent is used to partially break through the clot, following which clot-dissolving nanoparticles are released which completely break down the clot. The new treatment has a lower risk microclot formation when compared to stent-retriever thrombectomy procedures that are currently used.
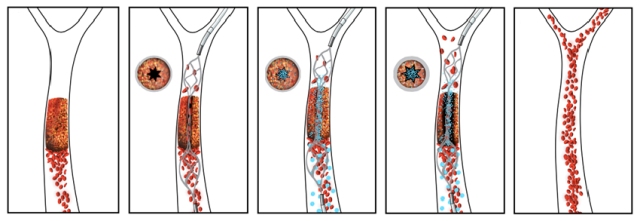 The new drug-device combination pairs an injectable clot-busting nanotherapeutic that targets blockages with an intra-arterial device that restores blood flow to obstructed vessels, as shown in this illustration.
The new drug-device combination pairs an injectable clot-busting nanotherapeutic that targets blockages with an intra-arterial device that restores blood flow to obstructed vessels, as shown in this illustration.
According to the new research the drug-device combination is capable of rapidly dissolving stroke-causing clots in blood vessels located in the brain. The new treatment incorporates an injectable clot-breaking nanotherapeutic with an intra-arterial stent to remove blockages and restore the flow of blood in clogged blood vessels.
The nanotherapeutic, developed at the Wyss Institute, consists of a cluster of biodegradable nanoparticles onto which a coating of a tissue plasminogen activator (tPA), a clot degrading drug, is applied.
The tPA coating effectively mimics the behaviour of blood platelets in our body. Clots restrict the passage of blood through vessels, resulting in an increase in the local blood pressure. The increase in pressure acts as a physical signal for that causes platelets to stick to the walls of the vessel. In a similar way, the nanotherapeutic also responds to an increase in local blood pressure by releasing tPA-coated nanoparticles which can then dissolve the clot.
Until now, mechanically activated nanotherapeutics have not been effective in treating total vascular blockages, i.e. blockages characterized by a complete cut-off of blood flow, a predominant cause of stroke. The most effective current method for stroke treatment is by a stent-retriever thrombectomy procedure. This procedure involves inserting a small tube through the blockage, with a closed stent is passed through it. The stent is then opened to drag the blood clot out of the vessel.
Even with the retriever thrombectomy procedure, not all clots can be removed with a successful outcome said. Clot fragments can be dislodged, which can lead to microclots and tissue damage downstream in the brain circulatory system, and physical dragging of the stent through the vessel can potentially be damaging as well.
Dr. Gounis - New England Center for Stroke Research
In the new approach, the stent is not used to pull out the clot; instead it is used to create a narrow channel at the centre of the clot that restores some, but not all, of the blood flow. The rush of blood through the small hole results in a high degree of shear force due to localised pressure. This acts as a stimulus for the nanotherapeutic, which discharges allowing the tPA to dissolve the entire clot.
The stent can then be re-sheathed and easily removed from the vessel after the clot is dissolved. In cases where fragments of the clot break off and are transported through the circulatory system the nanoparticles are still stuck to them and will continue to degrade the clot fragments locally.
What’s progressive about this approach is that the temporary opening of a tiny hole in the clot—using a stent device that is already commonly used clinically—results in a local rise in mechanical forces that activate the nanotherapeutic to deploy the clot-busting drug precisely where it can best do its job.
Dr. Ingber - Harvard School of Engineering and Applied Sciences.
The research will be published in the December edition of the journal, Stroke,