Jan 13 2016
A research team from the University of Amsterdam's Van’t Hoff Institute for Molecular Sciences (HIMS) has discovered a new method to achieve improved catalytic performance. In this method, self-assembled and functionalized nanospheres are used as ‘nanocentrators,’ enabling a very efficient catalytic conversion.
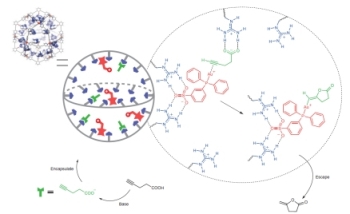 Schematic representation of the working principle of the nanoconcentrator. The gold(I) catalysts (drawn in red) are located in the sphere. Once the substrate (in black) is deprotonated the anionic substrate (in green) enters the sphere to pre-organize close to the catalyst via hydrogen bonding to the guanidine-binding site (displayed in blue). After rapid conversion of the substrate, the neutral cyclic product leaves the sphere. Image: HIMS.
Schematic representation of the working principle of the nanoconcentrator. The gold(I) catalysts (drawn in red) are located in the sphere. Once the substrate (in black) is deprotonated the anionic substrate (in green) enters the sphere to pre-organize close to the catalyst via hydrogen bonding to the guanidine-binding site (displayed in blue). After rapid conversion of the substrate, the neutral cyclic product leaves the sphere. Image: HIMS.
The research group, headed by Professor Joost Reek, have reported their findings in the Nature Chemistry journal.
Joost Reek’s ERC Advanced Grant funded the work, which was carried out as part of the UvA’s key research field “Sustainable Chemistry.” The working principle of natural enzymes was the source of inspiration for the new concept of catalytic nanospheres. They allow the binding of molecules in well-defined pockets, and within close proximity of their active sites. As a result, a pre-organization is introduced and highly efficient transformations are achieved. This enzymatic behavior was recreated by the research team in synthetic nanocontainers, which demonstrated an improvement of catalytic performance due to their very high local catalyst concentrations.
The new nanocontainers are formed by self-assembly, nano-sized spheres are formed by mixing 24 so-called ditopic nitrogen ligands and 12 palladium metals. The ligands are altered with guanidinium binding motifs in order for the resulting nanocontainers to bind carboxylates and sulfonates in their interior.
The employment of multiple binding sites, also known as cooperative binding, allows sulfonates to bind more firmly when compared to carboxylates. The sulfonated gold-based catalysts are firmly secured by this method, while the remaining binding sites are available for the pre-organisation of the carboxylate moieties that are to be converted (the substrates).
A gold-catalyzed cyclization reaction is used to establish the working principles of this ‘nanoconcentrator’ system. In a common system both the reactants and the catalyst are dissolved in a solvent, and not pre-organized. However, ‘nanoconcentrator’ systems show drastically improved reaction rates. This is due to the pre-organized substrate and very high local catalyst concentrations. Catalyst and substrate concentrations greatly increase the reaction rates, although unfavorable catalyst/reactant ratios or solubility problems generally limit this action. Nevertheless, the high local catalyst concentrations in the self-assembled nanospheres help resolve this issue.
In general many metal catalysts are used with sulfonate groups to make them soluble in water, whereas this nanoconcentrator system provides a widely applicable approach to various reactions. The researchers found that no or slow conversion of neutral (acid) substrates takes place with the encapsulated sulfonate-containing gold catalysts. This is the first step towards developing more complex catalyst systems with base-triggered on/off switching and substrate-selective catalysis.