Jan 25 2016
Scientists at Rice and Swansea Universities use a microwave for decontaminating carbon nanotubes. A typical microwave oven has been effective as part of a dual-step nanotube cleaning process, which has been invented by the scientists.
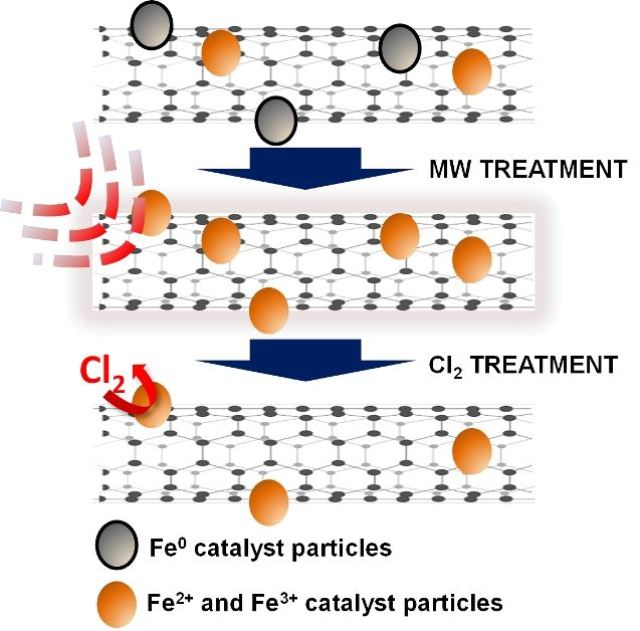 Treatment with a microwave oven and chlorine removes stubborn iron catalyst residues from carbon nanotubes, according to researchers at Rice University and Swansea University. The two-step process may make them more suitable for sensitive applications. (Credit: Virginia Goméz Jiménez/Swansea University)
Treatment with a microwave oven and chlorine removes stubborn iron catalyst residues from carbon nanotubes, according to researchers at Rice University and Swansea University. The two-step process may make them more suitable for sensitive applications. (Credit: Virginia Goméz Jiménez/Swansea University)
The common nanotubes can be used for forming microelectronic components or electrical fibers. For sensitive applications such as solar panels and drug delivery, the nanotubes have to be as pristine as possible.
Nanotubes are formed from metal catalysts in the presence of heated gas, but catalyst residues, such as iron, can get stuck inside and on the tubes. The residues cannot be removed easily by chemical or physical treatment, as the gas that is used for making the tubes allows carbon atoms to form. The atoms encapsulate layers and encircle the iron residues, reducing the effectiveness of their removal during purification. In the new process, the nanotubes are treated in a microwave oven to burn off the amorphous carbon. The tubes are then treated with chlorine at high temperature to remove the extraneous particles.
The new process has been published in the Royal Society of Chemistry journal, RSC Advances.
The labs of chemists Robert Hauge, Andrew Barron and Charles Dunnill worked on the project. Barron is a professorat Swansea University in the United Kingdom and at Rice in Houston. Hauge at Rice University is an expert in nanotube growth techniques. Dunnill is a senior lecturer at Swansea’s Energy Safety Research Institute.
There are several ways to purify nanotubes, but they all come at a cost,
The chlorine method developed by Hauge has the advantage of not damaging the nanotubes, unlike other methods,” he said. “Unfortunately, many of the residual catalyst particles are surrounded by a carbon layer that stops the chlorine from reacting, and this is a problem for making high-purity carbon nanotubes.
Andrew Barron, Professor, Swansea University
The scientists collected spectroscopy data, and microscope images on batches of multi-walled and single-walled nanotubes prior to, and after microwaving them in an oven (1000 W), and again after dipping them in chlorine gas under high pressure and heat. They realize that the iron particles when exposed to the microwave react with chlorine easier. The volatile iron chloride is then removed.
Eliminating iron particles lodged inside large multi-walled nanotubes is harder, but transmission electron microscope images showed their quantity was reduced, particularly in single-walled tubes.
We would like to remove all the iron, but for many applications, residue within these tubes is less of an issue than if it were on the surface. The presence of residual catalyst on the surface of carbon nanotubes can limit their use in biological or medical applications.
Andrew Barron, Professor, Swansea University
Co-authors of the study are Virginia Gomez, postdoctoral research assistant at Swansea; Silvia Irusta, a professor at the University of Zaragoza, Spain; and Wade Adams, a senior faculty fellow in materials science and nanoengineering at Rice.
Barron is the Charles W. Duncan Jr.–Welch Professor of Chemistry and a professor of materials science and nanoengineering at Rice and the Sêr Cymru Chair of Low Carbon Energy and Environment at Swansea. Hauge is a distinguished faculty fellow in chemistry and in materials science and nanoengineering at Rice.
The research was supported by the Robert A. Welch Foundation and the Welsh Government Sêr Cymru Program.