Aug 15 2016
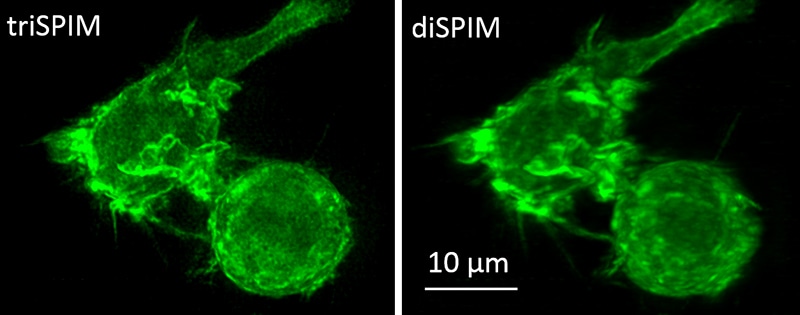 This is a macrophage actin labeled with green fluorescent protein, imaged with the new triSPIM microscope (left) and the diSPIM (right). (CREDIT: Yicong Wu and Valentin Jaumouille)
This is a macrophage actin labeled with green fluorescent protein, imaged with the new triSPIM microscope (left) and the diSPIM (right). (CREDIT: Yicong Wu and Valentin Jaumouille)
Taking inspiration from medical imaging, scientists have been motivated to develop a multi-view microscope capable of capturing higher-resolution, 3D images of live cells and tissues without the need for upping the dosage of potentially hazardous radiation received by the specimens.
The researchers worked collaboratively at the Marine Biological Laboratory’s Whitman Center, and they published their results in the August issue of the journal Optica.
Everybody knows fluorescence imaging is inefficient in that the microscope only captures a portion of the light (spewing off the specimen). In this paper, we showed you can not only capture that lost light, but use computation to fuse it to the existing image and make the image sharper.
Hari Shroff , National Institute of Biomedical Imaging and Bioengineering
Yicong Wu, a staff scientist in Shroff’s lab, developed the new system capable of obtaining a resolution of up to 235 x 235 x 340 nanometers, which is double the volumetric resolution of conventional fluorescence microscopy techniques.
The new microscope consists of three objective lenses, which acquires views of the samples simultaneously, allowing it to obtain more of the available light and providing additional details about the specimen. These views are then arranged and merged by a computational process called deconvolution.
The computations were all worked out by developing an alliance with co-author Patrick La Rivière of the University of Chicago’s Radiology Department, who generally develops algorithms for enhancing “dose efficiency” in human-scale medical imaging, such as CAT scans.
In medical imaging, we are always worried about dose, about capturing every X-ray [used on the patient to improve scan resolution]. We are concerned with ‘How can we do more with less?' If you use very intense illuminations to image something microscopic like a worm embryo, you might change its biology or even kill it. You need to be dose efficient with your light.
Patrick La Rivière, Radiology Department, University of Chicago
La Rivière and Shroff initially met at an MBL workshop on microscopy research in 2014. “On the plane [from Chicago], I read Hari’s paper about an earlier version of this microscope, and I saw he was using a very familiar, bread-and-butter deconvolution algorithm from the medical imaging world,” LaRivière says. “To me, that was the perfect entry point [into light imaging research]; it was something I knew. I chased Hari down after the workshop to see if he was open to collaboration, which he was.”
Financially aided by a University of Chicago-MBL Exploratory Research Fund award, the two started collaborating to enhance Shroff’s microscope, the diSPIM, which comprises two objective lenses, and eventually the newly developed three-lensed microscope called triSPIM.
This year La Rivière was named an MBL Fellow. Shroff is a Whitman Center Scientist and co-director of the MBL’s Optical Microscopy and Imaging in the Biomedical Sciences course.