Oct 18 2016
Scientists are pursuing a tiny solution for harnessing one of the world’s most abundant sources of clean energy: Water.
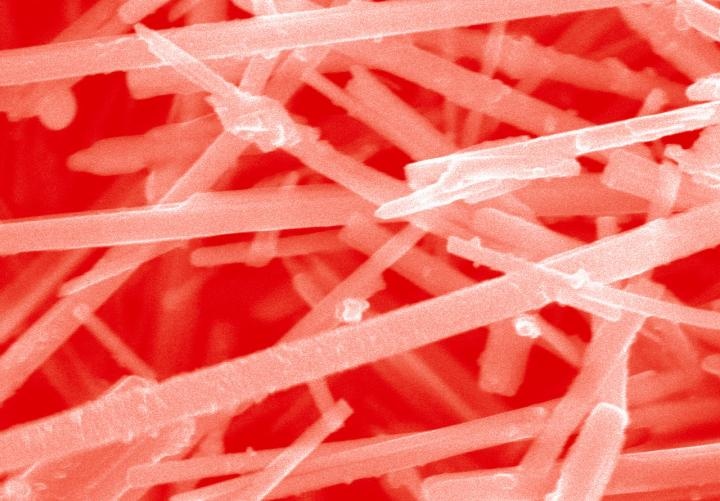 A scanning electron microscope image, with color added, shows vanadium oxide nanowires coated with tiny semiconductor crystals called quantum dots. This combination of materials has shown promise for splitting water into oxygen and hydrogen fuel, which could be used to power cars, buses, boats and other modes of transportation. Credit: Christopher Milleville
A scanning electron microscope image, with color added, shows vanadium oxide nanowires coated with tiny semiconductor crystals called quantum dots. This combination of materials has shown promise for splitting water into oxygen and hydrogen fuel, which could be used to power cars, buses, boats and other modes of transportation. Credit: Christopher Milleville
By marrying teeny crystals called quantum dots to miniature wires, the researchers are developing new materials that show promise for splitting water into oxygen and hydrogen fuel, which could be used to power cars, buses, boats and other modes of transportation.
“Hydrogen is seen as an important source of green energy because it generates water as the only byproduct when it’s burned,” says University at Buffalo chemist David Watson, PhD, one of the project’s lead researchers. “The hybrid materials we’re developing have the potential to support the cheap and efficient production of hydrogen gas.”
Watson, professor and chair of chemistry in the UB College of Arts and Sciences, has received a $550,000 grant from the National Science Foundation (NSF) to pursue the work. UB physics professor Peihong Zhang, PhD, is also a partner on the research, which is part of a larger $1.4 million NSF-funded project that teams UB with Texas A&M University, Binghamton University and Rensselaer Polytechnic Institute to develop the new catalysts for splitting water.
The project is funded through the NSF’s Designing Materials to Engineer and Revolutionize our Future program, which supports the White House’s Materials Genome Initiative for Global Competitiveness by accelerating the discovery of new materials.
A reaction powered by the sun
The materials under development are catalysts designed to harvest sunlight to drive the chemical reaction that divides water into oxygen and hydrogen.
They are formed from miniscule wires of vanadium oxide that are combined with various metal ions, then glazed with a coating of semiconductor quantum dots.
When they’re exposed to the sun, these hybrid materials generate two critical ingredients for splitting water: a free-floating electron and what chemists call an electronic hole (the absence of an electron where there would normally be one). Both the electron and the hole are used in the multi-step chemical reaction that converts water into oxygen and hydrogen gas.
So far, the research team successfully created materials that efficiently produce and separate both a free electron and a hole, though the scientists have yet to demonstrate that their hole can be used successfully in the water-splitting reaction.
From an industry perspective, the nanowire-quantum dot approach has benefits. Both the nanowires and quantum dots can be easily produced in large quantities from materials that are abundant in the crust of the earth, and both are “tunable,” Watson says. As he explains, changing the size of the quantum dots alters their electronic properties, as does combining the vanadium oxide nanowires with new materials. This makes it possible to tweak both components to maximize their efficiency at leveraging sunlight to split water.
“It’s a very flexible approach — a versatile platform for converting sunlight and water into fuel,” Watson says.
The scientists will use the new NSF funding to support an exploration of the best combinations of quantum dots and nanowires. In concert with synthesizing new materials, they will computationally predict which structures will have the best electronic properties.
“We are trying to put together some fairly complex machinery to mimic photosynthesis performed by plants, which use sunlight to split water and make energy. The machinery will be built from these nanowire and quantum dot blocks, using them almost as Legos,” says Sarbajit Banerjee, PhD, professor of chemistry at Texas A&M University. “Calculations performed on supercomputers will guide us on how to rapidly put these blocks together.”
The research was seeded by a Scialog grant from the Research Corporation for Science Advancement, an Arizona-based foundation devoted to the advancement of science.