Nov 8 2016
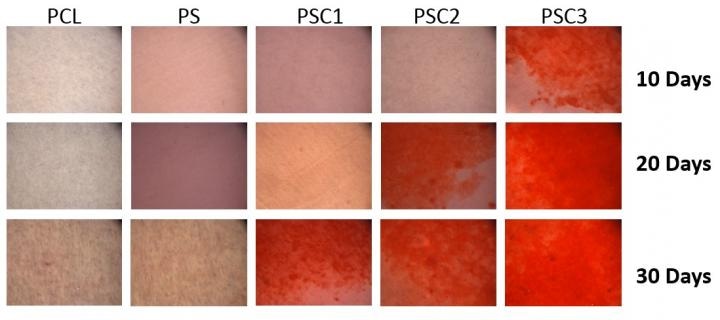 Dental follicle stem cells cultured on different scaffolds for 10, 20, and 30 days. Alizarin red stain was used to detect stem cell mineralization (the red indicates the bone mineralization). Bone differentiation rate of cells on the cellulose-nanocrystal scaffold was faster compared to the bioplastics scaffold. Credit: Martin Chiang, National Institute of Standards and Technology.
Dental follicle stem cells cultured on different scaffolds for 10, 20, and 30 days. Alizarin red stain was used to detect stem cell mineralization (the red indicates the bone mineralization). Bone differentiation rate of cells on the cellulose-nanocrystal scaffold was faster compared to the bioplastics scaffold. Credit: Martin Chiang, National Institute of Standards and Technology.
Imagine taking one of earth’s most abundant natural materials and extracting its strength to transform heavy objects into lightweight objects, to use as a substitute for synthetic materials, or to employ it in scaffolding to grow bone in oral health care, a rapidly growing area of science.
All this can be made feasible by using cellulose nanocrystals, which constitute the molecular matter of the entire plant kingdom. These nanocrystals can be mixed with plastics or other synthetic materials and used as industrial filler material. They are tough like glass, lightweight, green in color, and strong like steel.
Plastics are currently reinforced with fillers made of steel, carbon, Kevlar, or glass. There is an increasing demand in manufacturing for sustainable materials that are lightweight and strong to replace these fillers.
Cellulose nanocrystals are an environmentally friendly filler. If there comes a time that they’re used widely in manufacturing, cellulose nanocrystals will lessen the weight of materials, which will reduce energy.
Douglas M. Fox, associate professor of chemistry at American University
He has also submitted a patent for his study involving cellulose nanocrystals, in which their performance is improved by employing a simple, scalable technique. The results of his technique have been published in ACS Applied Materials and Interfaces, a chemistry journal. Fox’s technique enables the usage of cellulose nanocrystals not only as a biomaterial but also for various applications in infrastructure, transportation, and wind turbines.
The power of cellulose
Leaves, stems, and other organic materials in the natural world derive their strength from cellulose. This strength has already been extracted and used in various commercial materials. Cellulose fibers can be fragmented at the nano-level into tiny crystals having a diameter less than ten millionths of a meter. Scientists produce crystals of varying strengths and sizes by extracting cellulose from tunicate, i.e. ocean-dwelling sea cucumbers, wood, and particular types of bacteria, which are natural sources.
In spite of all the industry capacity, challenges are numerous. The tendency of nanocellulose to get dispersed within plastic urges researchers to find the precise amount of nanoparticle-matrix interaction that can produce the most lightweight and strongest property. Fox employed a simple ion exchange procedure to alter the nanocrystal surface chemistry, thereby overcoming the four main barriers. Ion exchange minimizes water absorption to prevent cellulose composites from losing their strength by absorbing water, enhances re-dispersal once the crystals are dried, reduces clumping, and increases the temperature at which the nanocrystals decompose, which is an important requirement to blend with plastics.
Cell growth
As a biomaterial, cellulose nanocrystals possess yet another commercial scope. In the field of dental regenerative medicine, for supporting a patient’s dental implants or teeth, sufficient bone volume ought to be restored. By collaborating with the National Institute of Dental and Craniofacial Research of the National Institutes of Health, scientists at the National Institute of Standards and Technology (NIST), are in the search for an enhanced clinical approach for regrowing a patient’s bone. Upon conducting experiments with Fox’s modified nanocrystals, the scientists were able to disperse the nanocrystals in scaffolds for the purposes of dental regenerative medicine.
When we cultivated cells on the cellulose nanocrystal-based scaffolds, preliminary results showed remarkable potential of the scaffolds for both their mechanical properties and the biological response. This suggests that scaffolds with appropriate cellulose nanocrystal concentrations are a promising approach for bone regeneration.
Martin Chiang, team leader for NIST’s Biomaterials for Oral Health Project
Fox has formed one more alliance with Owens Corning, a company specializing in fiberglass insulation and composites, as well as with Georgia Institute of Technology to study the advantages of substituting glass-reinforced plastic used in cars, airplanes and wind turbines. He is also working with NIST and Vireo Advisors for the characterization of the safety and health of cellulose nanofibers and nanocrystals.
As we continue to show these nanomaterials are safe, and make it easier to disperse them into a variety of materials, we get closer to utilizing nature’s chemically resistant, strong, and most abundant polymer in everyday products.
Douglas M. Fox, associate professor of chemistry at American University