Aug 7 2017
A new mathematical model of two different molecules reacting within so called nano reactors that serve as catalysts, has been developed by Theoretical Physicists. These Physicists achieved unexpected new insights as to what factors encourage reactions and how to control and choose them. The model is applicable for an extensive range of research fields, from Biophysics to Energy Materials.
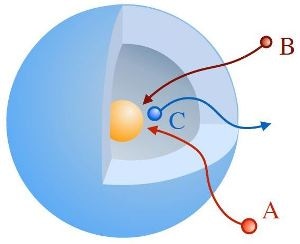 Sketch of an yolk-shell-type nanoreactor: reactands A and B diffuse through the shell and react to C at the catalytically active nanoparticle (yellow). Credit: HZB
Sketch of an yolk-shell-type nanoreactor: reactands A and B diffuse through the shell and react to C at the catalytically active nanoparticle (yellow). Credit: HZB
Nanoreactors are small systems that facilitate specific chemical reactions similar to what a catalyst does. Many are present in biological systems, such as certain proteins. However, Chemists are also capable of synthesizing artificial nanoreactors to control chemical reactions. A vital class of these nanoreactors has a “yolk & shell” architecture similar to an egg: a catalytically active metallic nanoparticle is surrounded by a shell comprising of a polymeric network. These types of nanoreactors can build isolated environments for specific reactions and limit them to the tiny space within the shell.
Mathematical description delivers new insights
We have now mathematically described for the first time how two different molecules are transported to react within nanoreactors. The new model shows clearly what factors favor a given reaction.
Dr. Rafael Roa, First Author of the publication in ACS Catalysis
Roa is First Author of the publication in ACS Catalysis and a Postdoc in the group led by Prof. Joe Dzubiella at the HZB Institute for Soft Matter and Functional Materials.
What matters most?
Some of the results are surprising: in contrast to what was expected, the reaction rate is not so much restricted by the concentration of the molecules in solution, but determinedly by the permeability of the nanoreactor’s shell.
This is extremely interesting since chemists today can often fine tune or even switch the permeability of these shells to specific molecules via variations in temperature or other parameters.
Dr. Won Kyu Kim, Co-Author
Photo-activation taken into account
The new model is a huge step forward from the older theory done many years earlier that could deal with only one molecule. Dzubiella states, “Our model is applicable to research on energy materials, and it can even take into account photo-activation of one of the molecules at the shell by sunlight”. With this work, he has accomplished one of the goals of his European Research Council (ERC) Consolidator Grant (2015-2020).
Predictions will be put to test
Dzubiella’s Soft Matter Theory group works in association with HZB Chemist Prof. Yan Lu, a recognized expert in synthetic nanoreactors. They are looking forward to test their theoretical predictions on real systems. “We’re now able to better understand what happens, and we expect to predict how the catalytic effects of these types of nanoreactors can be controlled – through feedback loops, for instance, which will stop or start the reaction at will.”
This work was funded by the ERC Consolidator Project “NANOREACTOR” of Prof. Dzubiella and results are published in ACS Catalysis (2017): “Catalyzed Bimolecular Reactions in Responsive Nanoreactors”. Rafael Roa, Won Kyu Kim, Matej Kanduč,Joachim Dzubiella, Stefano Angioletti-Uberti.