Oct 6 2017
Researchers at the Rice University have used individual nanoscale nuggets of aluminum, copper, gold, silver and similar metals—with the ability to tap energy of light and use it for various applications—and have found an innovative technique for developing multifunctional nanoscale structures.
 Nano-islands of ruthenium adhere to an aluminum nanoparticle. Rice University scientists and colleagues at the University of Cambridge combined aluminum nanoparticles and smaller metal particles as they created versatile plasmonic nanostructures. CREDIT: Image by Sadegh Yazdi.
Nano-islands of ruthenium adhere to an aluminum nanoparticle. Rice University scientists and colleagues at the University of Cambridge combined aluminum nanoparticles and smaller metal particles as they created versatile plasmonic nanostructures. CREDIT: Image by Sadegh Yazdi.
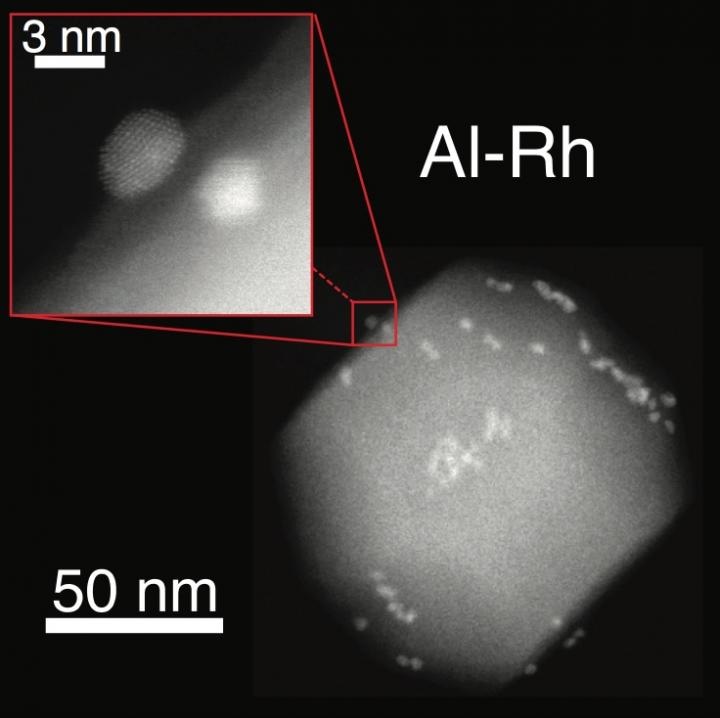
Apart from including an aluminum core, the novel structures are even dotted with smaller metallic islands. The materials have the ability to withstand localized surface plasmon resonances, that is, collective electron oscillations activated inside the nanostructure upon irradiating the particle by light.
Such nanoscale oscillations of electrons have the ability to activate chemical reactions and even stimulate reaction-promoting catalysts.
The method formulated in the labs of Emilie Ringe and Naomi Halas, Rice materials scientists, uses aluminum nanocrystals as a core for size-tunable transition metal islands that activate localized surface plasmon resonances. An earlier work performed by the teams of Halas and Ringe has shown that aluminum is known to be an effective plasmonic material, and the addition of smaller catalytic particles from three periodic table columns improves the ability of the structure to stimulate chemical reactions activated by the energy light.
According to the Scientists, the method paves the way for customizable surface chemistry as well as reactivity in a single material. It can be handy in the processes of surface-enhanced spectroscopy, photocatalysis and quantum plasmonics (i.e. the investigation of light’s quantum properties and the manner in which they interact with nanoparticles).
The study had been reported in the ACS Nano journal published by the American Chemical Society.
The Scientists stated that the general polyol method developed by them can be applied to blend a number of materials by using an uncomplicated and controllable procedure.
Dayne Swearer—Lead Author of the study and a Graduate Student at Rice—and his collaborators adopted a two-step synthetic technique, the first step of which is to reduce an aluminum precursor to synthesize purified aluminum particles with a width of 50-150 nm. Next, the purified aluminum particles are suspended in ethylene glycol, a metal salt precursor is added, and the solution is boiled to reduce the salts which ultimately nucleate and grow into nano-islands that adorned the surface of the original aluminum nanocrystals.
By means of an electron microscope, the Scientists discovered that a native aluminum oxide layer with a width of 2-4 nm divided the aluminum nanocrystal from the catalytic nano-islands. Moreover, the team worked in cooperation with Cambridge University Material Scientists Rowan Leary and Paul Midgley to adopt electron tomography to determine the location and size of over 500 individual ruthenium nano-islands formed on a single aluminum nanocrystal.
The naturally occurring nanoscale geometry of these new materials is really exciting. Because a thin layer of aluminum oxide separates the two materials, we can independently tune their properties to suit our needs in future applications.
Dayne Swearer, Lead Author of the study and Graduate Student, Rice University
The technique was applied in the lab to adorn aluminum nanocrystals with cobalt, iridium, iron, platinum, nickel, ruthenium rhodium and palladium. The width of the islands ranged from 2 to 15 nm.
According to Ringe, tailor-made devices coupling aluminum and plasmonic islands will enable easy activation of the most favored reactions.
In the year 2016, the research team demonstrated that palladium islands-adorned aluminum nanocrystals, synthesized by means of a distinctive technique, can be used for selective hydrogenations—unable to be carried out by simply heating in the dark—by irradiating with light.
We hope that with this new, expansive library of similar nanomaterials, many new types of previously inaccessible chemical reactions will become possible.
Dayne Swearer, Lead Author of the study and Graduate Student, Rice University
According to Swearer, the smaller size of the islands allows them to absorb light better than larger nanoparticles and also enables them to better produce hot electrons and introduce them into target molecules for initiating catalysis.
“The synthesis could be used to make even more elaborate combinations of metals and semiconductors from the periodic table,” explained Swearer. “Each new material combination has the potential to be explored for several applications.”
Rice Researchers Ryan Newell and Sadegh Yazdi; Graduate Students Hossein Robatjazi, Yue Zhang, and David Renard; and Peter Nordlander, a Professor of Physics and Astronomy, of Electrical and Computer Engineering, and of Materials Science and Nanoengineering, are the Co-authors of the paper. Leary is a Research Fellow and Midgley is a Professor of Materials Science at Cambridge University. Ringe and Halas are Co-advisors of Swearer.
Ringe is an Assistant Professor of Materials Science and Nanoengineering and of Chemistry. Halas is the Stanley C. Moore Professor of Electrical and Computer Engineering; a Professor of Physics and Astronomy, of Bioengineering, of Chemistry, and of Materials Science and Nanoengineering; and Director of the Laboratory for Nanophotonics and of the Smalley-Curl Institute.
The National Science Foundation, the Air Force Office of Scientific Research’s Multidisciplinary University Research Initiative, the Army Research Office, the Defense Threat Reduction Agency, the Welch Foundation, and the American Chemical Society Petroleum Research Fund together supported the study.
Aluminum nanoparticles with plasmonic islands
An animation shows the position of hundreds of ruthenium nano-islands on a single aluminum nanocrystal. Rice University scientists combined aluminum nanoparticles and smaller metal particles to create versatile plasmonic nanostructures. The technique allows for customizable surface chemistry and reactivity in one material.