Nov 14 2017
Researchers from RUDN University (Russia) have invented a new method for converting titanium nanoparticles into an efficient substance capable of removing toxic phenol present in water, even in visible light. The results of the study have been reported in the Journal of Materials Science: Materials in Electronics.
 Researchers from RUDN University (Russia) have come up with a new method to convert titanium nanoparticles into an efficient substance capable of removing toxic phenol from water, even in visible light. CREDIT Yahya Absalan
Researchers from RUDN University (Russia) have come up with a new method to convert titanium nanoparticles into an efficient substance capable of removing toxic phenol from water, even in visible light. CREDIT Yahya Absalan
Environmental pollution is arguably one of the greatest threats to humanity -- and to the whole planet Earth. Industry is responsible for many types of pollution, and the transfer of toxic organic substances into the water is one of them.
Yahya Absalan, Ph.D Student, RUDN and the Key Author of the Paper
One such substance is phenol (and its derivaties), produced on a huge scale (about 7 billion kilos per year). Phenol derivatives behave as precursors to many compounds and materials, detergents, plastics and pharmaceuticals first of all. However, this substance may lead to harmful effects on the central nervous system, heart, kidneys and liver.
Chemists have elaborated quite a few methods that help remove phenol from water. One of these methods employs nanomaterials, valued for their magnetic and optical properties. These properties are derived from unique features of nanoparticles because of their surface activity and large surface compared with material's bulk phase.
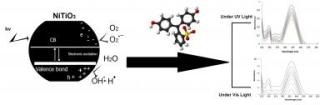
The researchers from RUDN University (general chemistry, colloidal chemistry, physical and inorganic chemistry departments) work with titanium dioxide (TiO2) nanoparticles. This is a semiconductor that is capable of reacting with water after absolving UV-light. This reaction results in the generation of two radicals (OH• and O2-•), that, in turn, could react with phenol and then lower the concentration of phenol in water.
However, there is a problem: energy band gap of dioxide titanium is 3.25, so it may only absorb UV-light (and, thus, purify water only under extremely costly and specific conditions). The RUDN chemists tried to alter dioxide titanium with transition metals - any of metallic elements occupying a central block (Groups IVB-VIII, IB, and IIB, or 4-12) in the periodic table. Pure MTiO3 ("M" here stands for "metal", cadmium, chrome, nickel, manganese, iron and cobalt) nanoparticles were developed when the precursor was heat-treated at 750 °C for 210 minutes.
Doping a transition metal into dioxide titanium enables reducing energy band gap and, consequently, increases the wave length. New substance (TiO3) can absorb not only UV-light, but also visible light. This would allow removal of phenol from water in non-specific conditions - almost everywhere.
The researchers have earlier planned to expand the scope of their experiments - to employ rare metals instead of standard, for example.
There is still a long way to go before we could transfer our new method to industry. Six months, or two years, perhaps. Anyway, this is a very promising substance to be used against pollution.
Yahya Absalan, Ph.D Student, RUDN and the Key Author of the Paper