Mar 27 2019
At Duke University, materials scientists have hypothesized a novel “oil-and-vinegar” method for creating self-assembling materials of extraordinary architectures made from sphere-shaped nanoparticles.
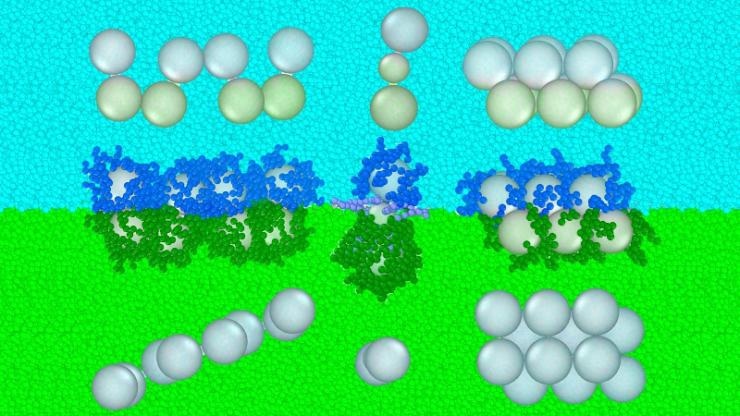 Researchers have created a new ‘oil and vinegar’ approach to forming nanoparticle structures. In this conceptual model, green and blue elements repel one another. Not only does this create a boundary layer where particles tend to congregate, researchers can attach molecules to individual nanoparticles to make them more or less repulsed by an individual layer. This approach is depicted across the center of the image, while the resulting structures can be seen from different angles above and below. (Image credit: Gaurav Arya, Duke University)
Researchers have created a new ‘oil and vinegar’ approach to forming nanoparticle structures. In this conceptual model, green and blue elements repel one another. Not only does this create a boundary layer where particles tend to congregate, researchers can attach molecules to individual nanoparticles to make them more or less repulsed by an individual layer. This approach is depicted across the center of the image, while the resulting structures can be seen from different angles above and below. (Image credit: Gaurav Arya, Duke University)
The ensuing structures can have significant implications in optics, electronics, plasmonics, multi-stage chemical catalysis, and other associated applications. The latest method was reported online on March 25th, 2019 in the journal, ACS Nano.
A system of suspended spherical nanoparticles typically clumps together. When left to their own tendencies, these nanoparticles will attempt to increase their points of contact by sandwiching themselves as closely as possible. This leads to the formation of a 3D crystalline structure or arbitrary clusters.
Conversely, materials scientists usually prefer to construct structures that are more open and have lower dimensions, like sheets or strings, in order to manipulate specific phenomena that can take place in the voids between different kinds of particles. Moreover, materials scientists invariably look for ingenious ways to accurately manage the placements and sizes of those particles and spaces.
In the latest work, Gaurav Arya, an associate professor of mechanical engineering and materials science at Duke University, has recommended a technique that manipulates the layers created by liquids. Similar to a bottle of vinaigrette left on the shelf for an extended period of time, these layers refuse to combine together.
When placed into such a system, spherical nanoparticles tend to form one layer at the interface of the opposing liquids; however, they do not have to necessarily remain there. By adhering “vinegar” or “oil” molecules to the surfaces of the particles, scientists can make these particles to float more on one side of the separating line than the other.
The particles want to maximize their number of contacts and form bulk-like structures, but at the same time, the interface of the different liquids is trying to force them into two layers. So you have a competition of forces, and you can use that to form different kinds of unique and interesting structures.
Gaurav Arya, Associate Professor, Department of Mechanical Engineering and Materials Science, Duke University
Arya’s concept is to accurately regulate the amount that every sphere-shaped nanoparticle is resisted by one liquid or the other. Based on Arya’s calculations, materials scientists can change this property along with others, like the size and composition of nanoparticles, and eventually make all kinds of fascinating shapes, ranging from zig-zag structures to spindly molecule-like structures, in which only a pair of nanoparticles contact at a time. It is even possible to imagine a number of varied layers functioning together to organize a system of spherical nanoparticles.
According to the proof-of-concept study, the nanoparticles can possibly be designed from anything. Electrical and plasmonic devices can benefit from semiconductors or gold, while other metallic elements can catalyze numerous chemical reactions. In the meantime, the opposing substrates forming the interface are modeled after many types of polymers that may also be utilized in such kinds of applications.
So far in this paper, we have only introduced the assembly approach and demonstrated its potential to create these exotic arrangements that you wouldn’t normally get. There are so many more things to do next. For one, we’d like to explore the full repertoire of possible structures and phases researchers could make using this concept. We are also working closely with experimentalists to test the full capabilities of this approach.
Gaurav Arya, Associate Professor, Department of Mechanical Engineering and Materials Science, Duke University
The National Science Foundation (CMMI Award 1636356, ACI-1053575) supported the study.