Mar 25 2021
Experimental flu shots newly developed by researchers safeguard animals from an extensive range of pandemic and seasonal influenza strains. At present, the vaccine product is being developed further toward clinical testing.
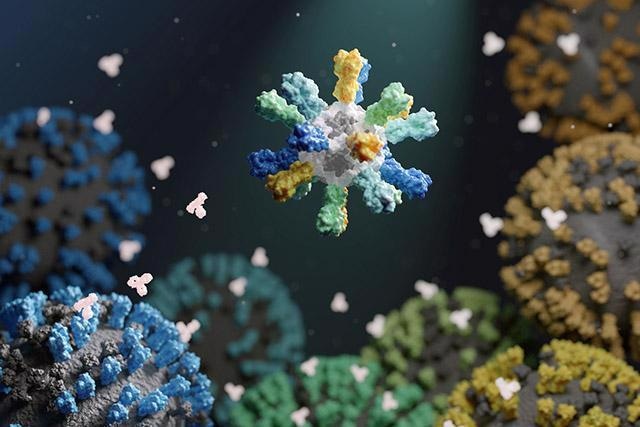 Depiction of a nanoparticle vaccine that contains proteins from many different flu strains. Image Credit: Institute for Protein Design.
Depiction of a nanoparticle vaccine that contains proteins from many different flu strains. Image Credit: Institute for Protein Design.
If these next-generation influenza vaccines are proven to be safe and effective, they might be an alternative to existing seasonal options by protecting against several more strains that existing vaccines do not cover sufficiently.
A paper describing how the new flu vaccines were developed and how they safeguard ferrets, nonhuman primates, and mice has been published in the March 24th edition of the Nature journal. This study was headed by scientists from the University of Washington School of Medicine (UW School of Medicine) and the Vaccine Research Center part of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Influenza virus results in a predicted death rate of 290,000 to 650,000 annually. Already existing flu vaccines, which should be taken on a seasonal basis, usually fail to safeguard against several circulating flu strains causing illness, and the risk of another influenza pandemic is imminent.
Most flu shots available today are quadrivalent, meaning they are made from four different flu strains. Each year, the World Health Organization makes a bet on which four strains will be most prevalent, but those predictions can be more or less accurate. This is why we often end up with ‘mismatched’ flu shots that are still helpful but only partially effective.
Daniel Ellis, Study Lead Author, University of Washington School of Medicine
Ellis is also a research scientist in the laboratory of Neil King, who is an assistant professor of biochemistry at the UW School of Medicine and a researcher at the Institute for Protein Design at UW Medicine.
The researchers made enhanced influenza vaccines by fixing hemagglutinin proteins from four different influenza viruses to tailor-made protein nanoparticles. This method allowed an unparalleled level of control on the molecular configuration of the consequent vaccine and provided an improved immune response than traditional flu shots.
The new nanoparticle vaccines, which consisted of the same four hemagglutinin proteins of commercially available quadrivalent influenza vaccines, evoked neutralizing antibody responses to vaccine-matched strains that were superior to or same as the commercial vaccines in nonhuman primates, ferrets, and mice.
Moreover, the nanoparticle vaccines—and not the commercial vaccines—induced defensive antibody responses to viruses that were not included in the vaccine formulation. These include avian influenza viruses H7N9 and H5N1, which are regarded as pandemic threats.
The responses that our vaccine gives against strain-matched viruses are really strong, and the additional coverage we saw against mismatched strains could lower the risk of a bad flu season.
Daniel Ellis, Study Lead Author and Research Scientist in Laboratory of Neil King, University of Washington School of Medicine
This study was financially supported by the National Institutes of Health (R01GM120553, HHSN272201700059C as well as intramural funding to the Vaccine Research Center), Open Philanthropy Project, Audacious Project, Burroughs Wellcome Fund, University of Washington Arnold and Mabel Beckman cryo-EM center, and a Pew Biomedical Scholars Award.
Journal Reference:
Boyoglu-Barnum, S., et al. (2021) Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. doi.org/10.1038/s41586-021-03365-x.