A long-time dream of several researchers and health care providers has been to create nanoscale computers for precision health care.
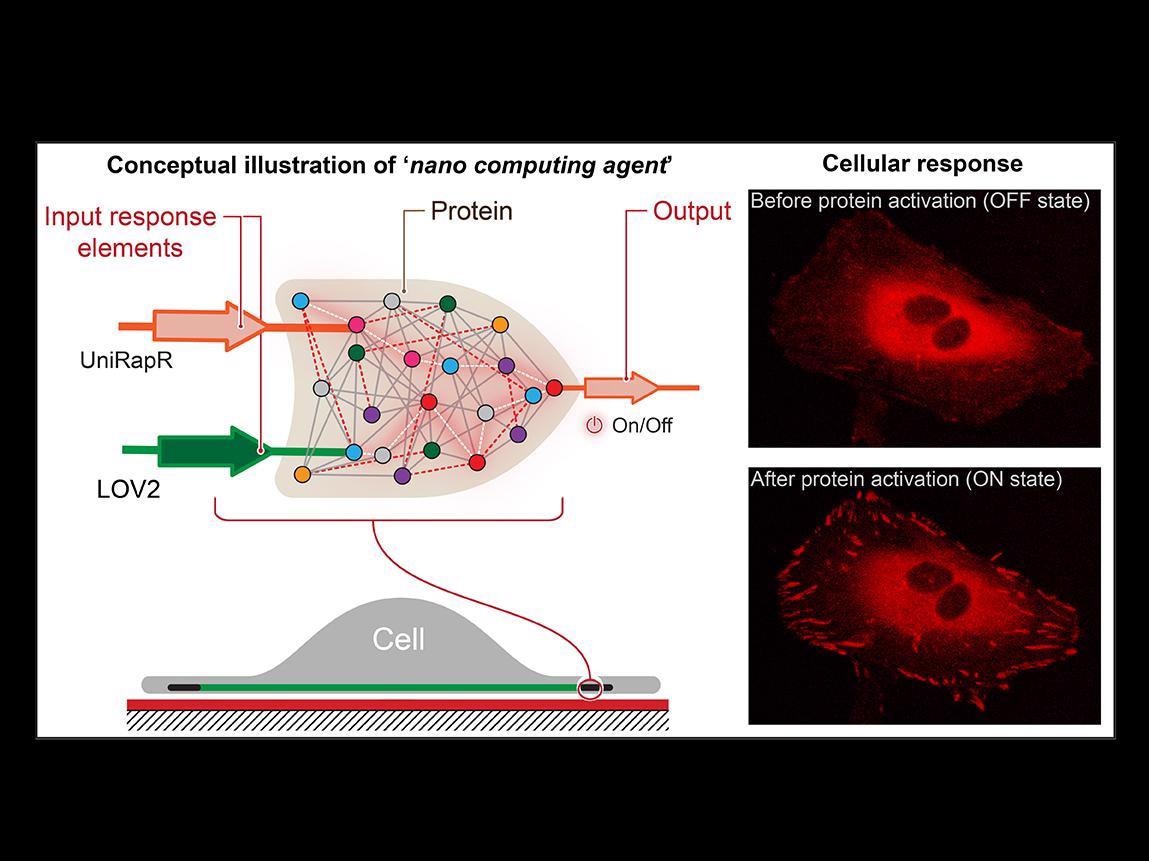 Researchers created a transistor-like “logic gate,” which is a type of computational operation in which multiple inputs control and output, and embedded it into a protein. They found that not only could they rapidly activate the protein using light and the drug rapamycin, but also that this activation resulted in the cells undergoing internal changes that enhanced their adhesive capabilities, which ultimately decreased their motility. Image Credit: Yashavantha Vishweshwaraiah, Penn State.
Researchers created a transistor-like “logic gate,” which is a type of computational operation in which multiple inputs control and output, and embedded it into a protein. They found that not only could they rapidly activate the protein using light and the drug rapamycin, but also that this activation resulted in the cells undergoing internal changes that enhanced their adhesive capabilities, which ultimately decreased their motility. Image Credit: Yashavantha Vishweshwaraiah, Penn State.
At present, for the first time ever, scientists at Penn State have produced a nanocomputing agent that has the ability to regulate the function of a specific protein involved in cancer metastasis and cell movement. The study sets the stage for the construction of complicated nanoscale computers for the prevention and treatment of cancer and other diseases.
Nikolay Dokholyan, G. Thomas Passananti Professor, Penn State College of Medicine and his colleagues — including Yashavantha Vishweshwaraiah, postdoctoral scholar in pharmacology, Penn State — have designed a transistor-like “logic gate.” It is a kind of computational operation in which several inputs control an output.
Our logic gate is just the beginning of what you could call cellular computing. But it is a major milestone because it demonstrates the ability to embed conditional operations in a protein and control its function. It will allow us to gain a deeper understanding of human biology and disease and introduces possibilities for the development of precision therapeutics.
Nikolay Dokholyan, G. Thomas Passananti Professor, College of Medicine, Penn State
The logic gate includes two sensor domains that have been developed to respond to two inputs — light and the drug rapamycin. The team targeted the protein focal adhesion kinase (FAK) since it is involved in cell adhesion and movement, which are considered to be the initial steps involved in the development of metastatic cancer.
First, we introduced a rapamycin-sensitive domain, called uniRapr, which the lab had previously designed and studied, into the gene that encodes FAK. Next, we introduced the domain, LOV2, which is sensitive to light. Once we optimized both domains, we combined them into one final logic-gate design.
Yashavantha Vishweshwaraiah, Postdoctoral Scholar in Pharmacology, Penn State
The modified gene was inserted into HeLa cancer cells by the team through confocal microscopy, observed in the cells in vitro. They studied the impacts of each of the inputs individually, as well as the integrated effects of the inputs, on the behavior of the cells.
The researchers found not only that they were able to quickly trigger FAK with the help of light and rapamycin but also that this activation led to the cells experiencing internal variations that improved their adhesive capabilities. Eventually, this reduced their motility.
Their study was recently reported in the Nature Communications journal on November 16th, 2021.
We show for the first time that we can build a functioning nanocomputing agent within living cells that can control cell behavior. Also discovered some interesting features of the FAK protein, such as the changes it triggers in cells when it is activated.
Yashavantha Vishweshwaraiah, Postdoctoral Scholar in Pharmacology, Penn State
Dokholyan added that the team intends to eventually test such nanocomputing agents in vivo inside the living organisms.
The other authors from Penn State include Jiaxing Chen, graduate student; Venkat R. Chirasani, postdoctoral fellow; and Erdem D. Tabdanov, assistant professor of pharmacology. The study was financially supported by the National Institutes of Health and the Passan Foundation.
Journal Reference:
Vishweshwaraiah, Y. L., et al. (2021) Two-input protein logic gate for computation in living cells. Nature Communications. doi.org/10.1038/s41467-021-26937-x.