Multi-walled carbon nanotubes (MWCNTs) can be utilized for hydrogen storage as they are cost-effective, environmentally acceptable, and exhibit excellent performance in adsorption and desorption processes. An article published recently in the journal Heliyon describes a novel technique for the purification of multi-walled carbon nanotubes for H2 storage purposes.

Study: Hydrogen storage in purified multi-walled carbon nanotubes: gas hydrogenation cycles effect on the adsorption kinetics and their performance. Image Credit: petrmalinak/Shutterstock.com
Hydrogen; an Eco-Friendly Energy Source
Safe and renewable energy has become a focus of economic prosperity in developed countries. The primary objective is to satisfy the need for increased energy supply, which results from both population expansion and environmental concerns throughout the globe. As an environmentally safe energy source, hydrogen has the potential to be a cost-effective option for meeting rising energy demands. However, the three different forms of hydrogen, including compressed gas, cryogenic liquid, and solid-state form, provide significant storage and transportation difficulties.
Metallic hydrides, chemical hydrogen storage devices, and CNTs have all been highlighted as promising technologies for hydrogen storage in recent years. Due to financial and safety issues, several studies have focused on the storage of H2 in solid-state form, in which hydrogen mixes with metals and alloys by adsorption and desorption, as opposed to liquid-state storage.
The Use of Carbon Nanotubes (CNTs) for H2 Storage
The development of high hydrogen storage capabilities in carbon nanomaterials such as graphene oxide and CNTs has increased the focus on H2 storage in solid materials. Carbon nanotubes (CNTs) are excellent H2 storage materials because of their large surface area and small mass concentration. Adsorption and desorption are two very effective methods for storing hydrogen in carbon-containing solids.
However, recent investigations have revealed that the H2 storage capability of nanotubes is less than the criterion established by the United States Department of Energy (DoE). This is because carbon nanotube growing techniques and circumstances significantly impact the adsorption and desorption processes, resulting in poor storage capacity.
Contaminants such as metallic catalysts and carbonaceous elements are present in the final product due to synthesis processes used for making CNTs.
Purification of Multi-Walled Carbon Nanotubes
This paper presents the results of several procedures used to analyze multi-walled CNTs synthesized and purified by aerosol-aided chemical vapor deposition. The architecture, surface morphology, degree of graphitization, and quality of the nanotubes were all investigated. By using quartz crystal microbalance (QCM) technology, the H2 storage capacity and adsorption ability of CNTs were determined.
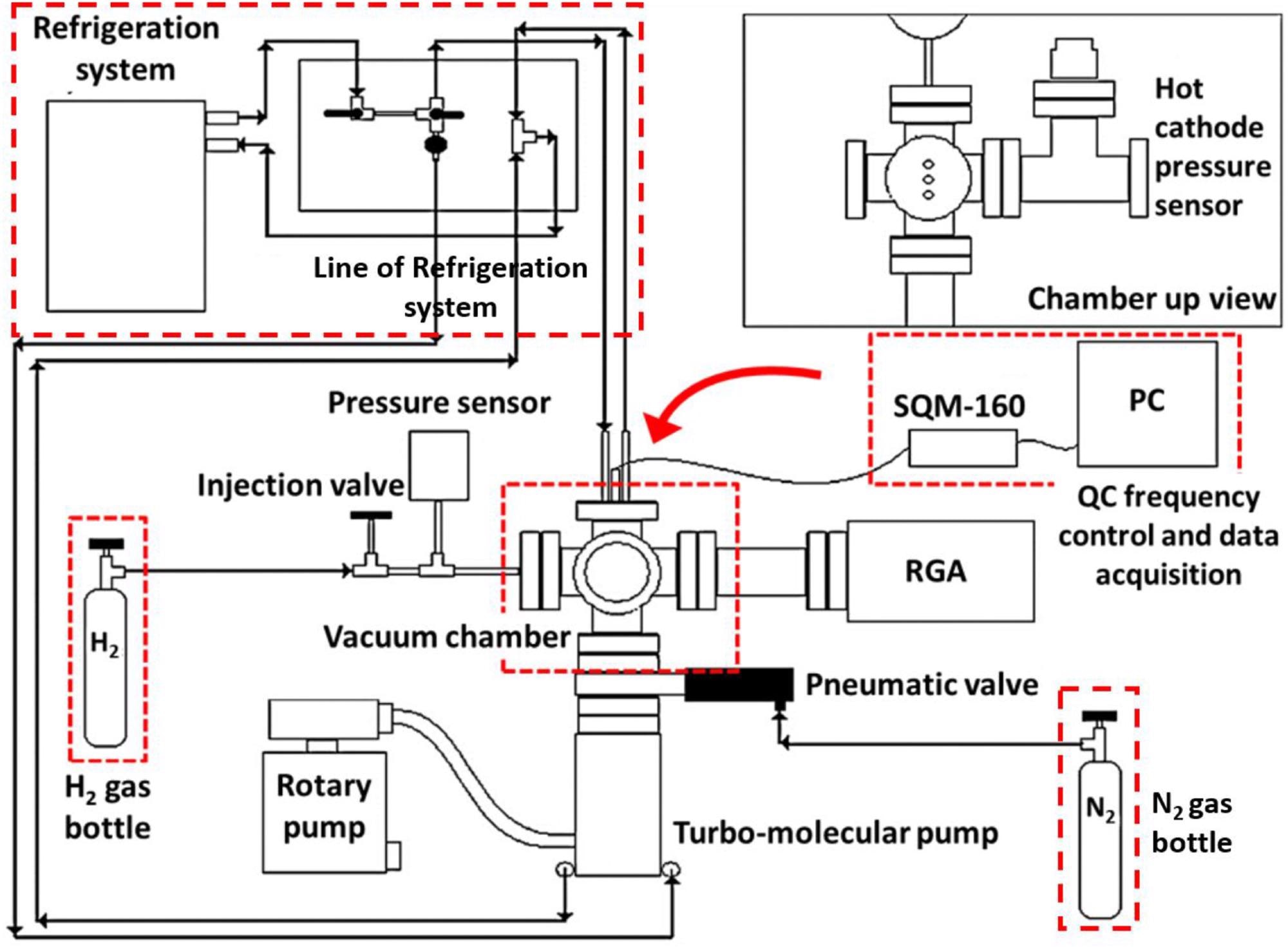
Scheme of the experimental set up for the H2 adsorption studies. Image Credit: Mosquera-Vargas, E., et al.
The approach of aerosol-assisted CVD was used to generate multi-walled CNTs in an aqueous solution. Following natural cooling of the system to ambient temperature, the darkened particles were collected for characterization. To examine the influence of purification of the MWCNTs on the secondary phases remaining and H2 storage capabilities, the materials were purified using hydrofluoric acid (HF) and hydrochloric acid (HCl).
Research Findings
The approach of aerosol-assisted CVD (AACVD) was used to generate multi-walled Purified multi-walled carbon nanotubes were used in this study to significantly enhance hydrogen storage capabilities. According to XRD analysis, the metal catalysts and carbon nanofibers in purified CNTs were separated and decomposed by thermal processing accompanied by an acid procedure.
The 15% pure carbon nanotube specimen had the highest specific surface area, which was nearly ten times greater than the other materials. According to Raman tests and TEM examinations, the purified MWCNTs contain minimal flaws and a crystalline structure in the graphene sheets.
Results also indicated that pure MWCNTs have outstanding hydrogen adsorption properties since they can adsorb more H2 molecules than un-purified carbon nanotubes with a perfect hexagonal lattice. The synthesis circumstances also influence the shape and storage capacity of CNTs and the purification method utilized.
Using the multi-walled CNT purification approach, the hydrogen storage capacity was shown to be relatively good at higher pressures. However, the H2 storage capability of purified carbon nanotubes at low (standard) pressure fell short of the standards set by the US DOE for static and mobile systems. At 12.79 kPa of hydrogen absorption pressure, the optimum result for H2 storage capability was determined to be 3.48 wt percent. However, this novel purification technique can pave the way for further research in the area of CNT usage for hydrogen storage applications.
Reference
Mosquera-Vargas, E., et al. (2021) Hydrogen storage in purified multi-walled carbon nanotubes: gas hydrogenation cycles effect on the adsorption kinetics and their performance. Heliyon, e08494. Available at: https://www.sciencedirect.com/science/article/pii/S2405844021025974
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.