Using nano-scale graphene oxide (sGO) modified nanopillars on microgroove hybridized polymeric array (NMPAs) can successfully regulate cellular differentiation in skeletal muscle fibers, research published in Nano Convergence has found.
Study: Nano-sized graphene oxide coated nanopillars on microgroove polymer arrays that enhance skeletal muscle cell differentiation. Image Credit: Jose Luis Calvo/Shutterstock.com
Sequenced laser interference lithographic processes and micro imprint etching were used to create sGO-coated NMPA (sG-NMPAs). As Polydimethylsiloxane (PDMS) has limited adhesive capability, researchers altered the well-known cytophilic nanomaterial graphene oxide (GO) used in this study.
Notably, mouse myoblast cells were successfully used for the cellular development of skeletal muscle fibers on the hybridized patterns, thanks to the micro-scale line sequences that direct cellular architecture into a lengthened form and the inclusion of sGO-coated nanomaterials.
As a result, the proposed sG-NMPAs have the potential to serve as a framework for quick and effective test-tube-based muscle cell growth.
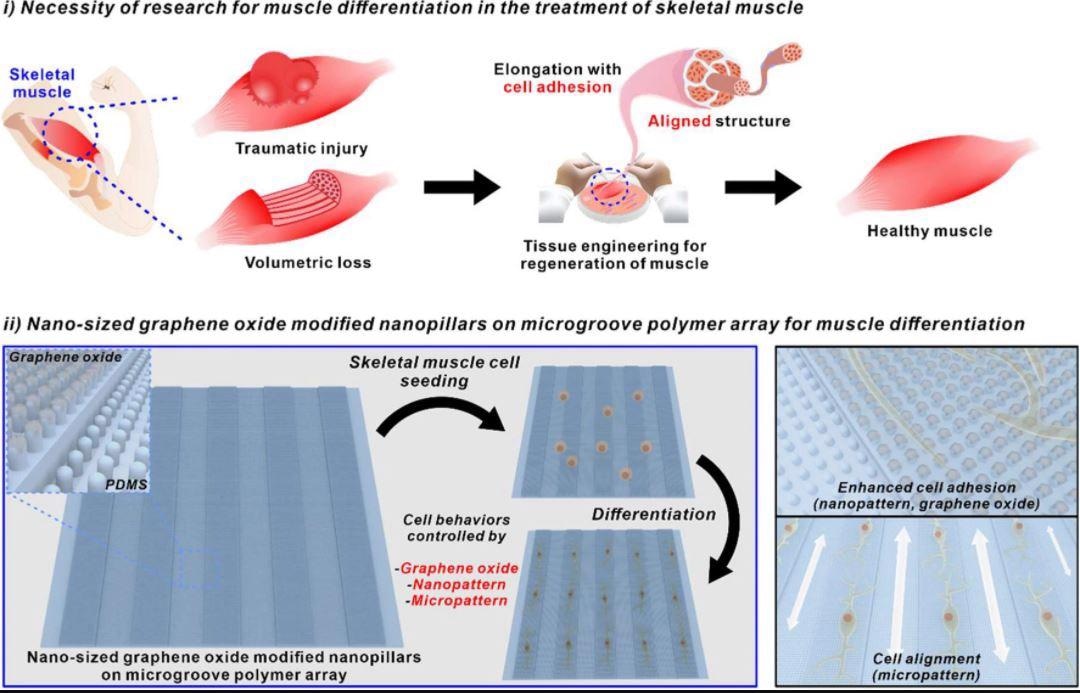
Schematic diagram of necessity of research for muscle differentiation in the treatment of skeletal muscle and nano-sized graphene oxide-modified nanopillars on microgroove polymer array for muscle differentiation. Image Credit: Choi, H., Kim, C., Lee, S. et al.
Regeneration of Skeletal Muscles
Skeletal muscles are a fundamental part of our bodies that regulate the majority of gestures and motions of the body.
When muscle fibers are injured locally, they may heal themselves via a series of chronological stages that include cellular mitosis, merging with preexisting muscle tissue fibers, and concluding repairs. However, in the absence of extrinsic regenerative stimuli, the loss of muscle mass or impairments caused by genetic defects cannot be recovered automatically.
To solve this problem, in vitro creation of muscle cells for transplant or drug testing has been identified as a potential strategy, primarily by regulating the development of pluripotent stem cells or unipotent myoblasts.
Regulating the external extracellular matrix has been shown to be efficient in directing muscle development among the numerous strategies described to restore skeletal muscle fibers.
Given that skeletal muscle tissues keep lengthened structures to provide linear forces, modifying cellular architecture into a lengthened form via the microgroove arrangement is advantageous for effective muscle cell growth.
Several microtextured structures composed of different components, such as metals, proteins, silicons, peptides, and biocompatible inorganic polymers, have been documented. All of them have been shown to influence cellular form with a large aspect ratio.
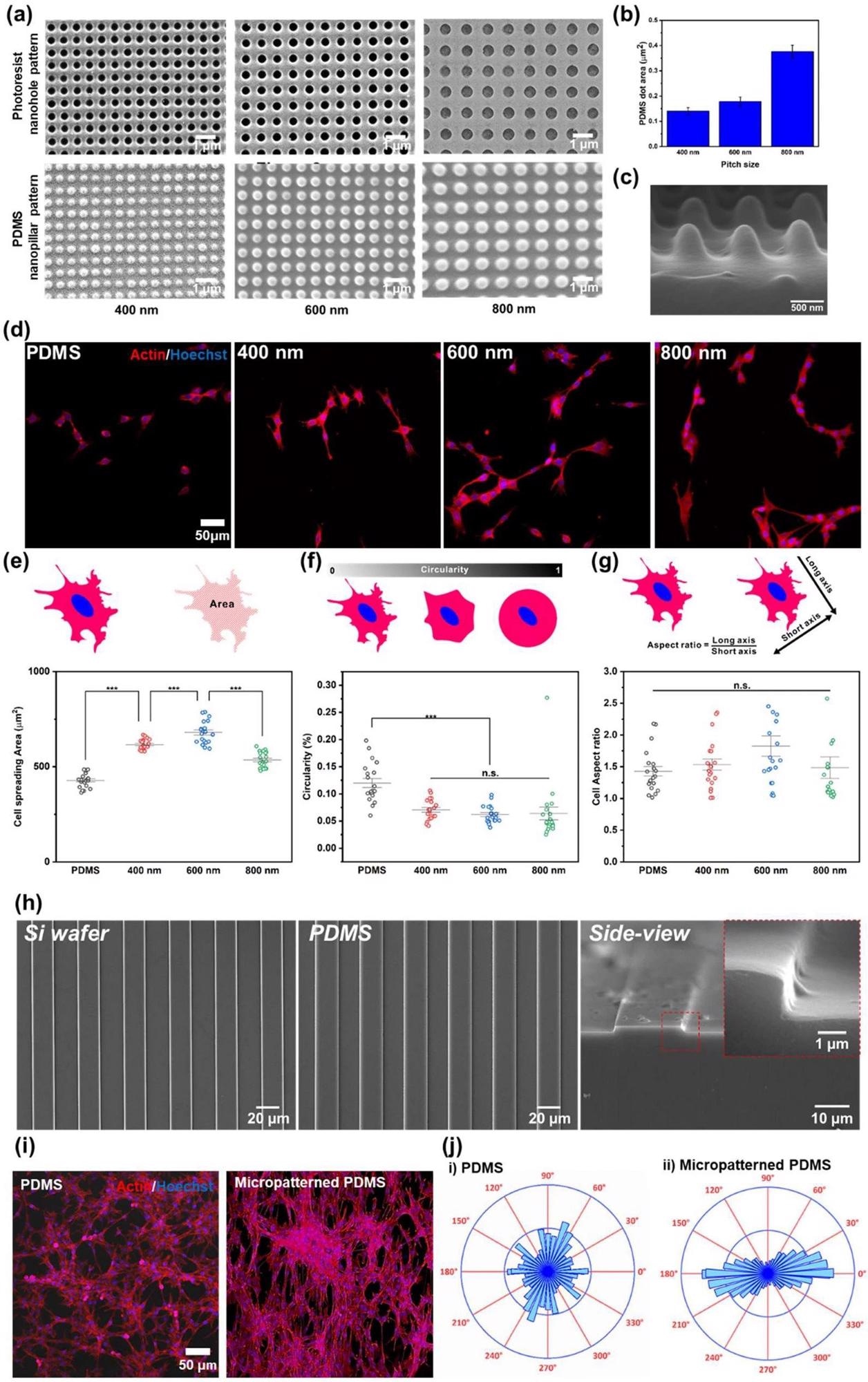
Confirmation of the effect of nano- and micropatterns for cell behaviors. a FE-SEM images of PR nanohole patterns and PDMS nanopillar patterns, and b size distribution of PDMS patterns. c 3D SEM image of PDMS nanopillar patterns. d Confocal microscope images of the cells on bare PDMS, and 400, 600, and 800 nm PDMS patterns (left to right) immunostained with actin (Red) and Hoechst (Blue). e−g Cell spreading area (e), circularity (f), and cell aspect ratio (g) of the cells on bare PDMS, and 400, 600, and 800 nm PDMS pattern. h FE-SEM images of micropatterned silicon wafer, PDMS and side view image of micropatterned PDMS. i Confocal microscope images of the cells on bare PDMS and micropatterned PDMS immunostained with actin (Red) and Hoechst (Blue). j Cell orientation analysis of the cells on the bare PDMS and micropatterned PDMS. (*** p ≤ 0.001). Image Credit: Choi, H., Kim, C., Lee, S. et al.
Inorganic polymeric composites (such as polydimethylsiloxane, polyvinyl alcohol, and polycaprolactone) are especially beneficial since they are inexpensive, innocuous to cells, and, most significantly, can be readily produced into a variety of shapes using a straightforward casting procedure.
Still, a fundamental disadvantage of such polymers is their water-repellent characteristic, limiting cellular attachment to substrate surfaces. As a result, including utility into the polymer-microtextured substrate is critical for its usage as a growth substrate to create skeletal muscle cells with higher conversion efficiency.
Fabrication of Nanoscale GO-Coated Nanopillars
A novel platform for effective skeletal muscle cell development has been developed, including nanopillar grids, nano-scale graphene oxide (sGO), and micro-grooves.
To manufacture sequential homogenous nanopore designs, the laser interference lithographic method was initially applied to a photo-resistant (PR)-coated silicon micropattern mold.
Reverse recreation of the microstructure involved the polydimethylsiloxane (PDMS) polymer being applied to the nanopore-modified silicon mold, resulting in the formation of NMPAs.
In addition to the nanopatterns, distinct-sized oxides of graphene were applied and deposited on the substrate to improve cell adherence on the microtextured polymeric arrays.
The efficacy of the produced hybrid arrays to create elongated cell shape was evaluated using three separate factors, including the myoblast cell line's cellular spread, circularity, and aspect ratio. Finally, immunological fluorescence imaging assessed the efficacy of the cellular development of skeletal muscles on various platforms.
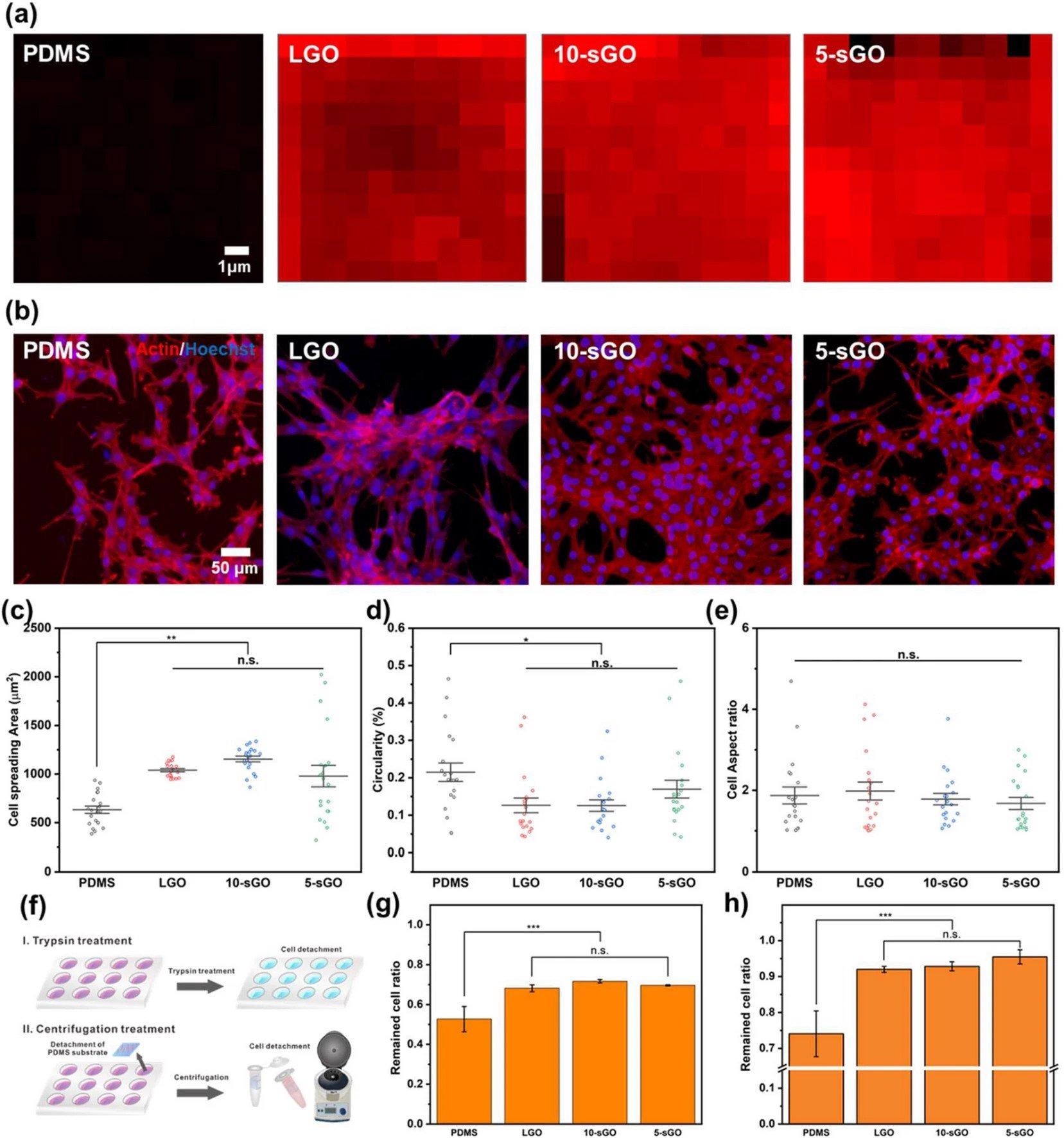
Confirmation of the effect of graphene oxide on cell behaviors. a FE-SEM images of PR nanohole patterns and PDMS nanopillar patterns, and b size distribution of PDMS patterns. Raman intensity map of the bare PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS. b Confocal microscope images of the cells on bare PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS immunostained with actin (Red) and Hoechst (Blue). c−e Cell spreading area (c), circularity (d), and cell aspect ratio (e) of the cells on bare PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS. f Schematic diagram of trypsin and centrifugation treatment process. g, h Cell ratio remaining on the PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS after trypsin (g) and centrifugation (h) treatment. (* p ≤ 0.5, ** p ≤ 0.01, *** p ≤ 0.001). Image Credit: Choi, H., Kim, C., Lee, S. et al.
Key Findings and Future Prospects
In this study, a graphene oxide-coated NMPA that promotes the cellular development of skeletal muscles was created.
Among the different diameters of GO tested, sGO (smaller than 10 nm) proved the most powerful. Overall, the hybridized pattern array improved cell adhesiveness and cellular spreading region, as well as cellular orientation.
Myogenic differentiation of cells on the hybridized pattern array was increased, similar to cell behaviors on the hybrid pattern array, when compared to the bare PDMS substrate.
The sGO on the hybrid pattern array improved cell differentiation and stable culture on the polymer substrate simultaneously.
The findings suggest that the proposed sG-NMPA is a suitable platform for the cellular development of skeletal muscles on a solid polymeric substrate. The sG-NMPA could be used in medicinal regeneration of cells, which involves cellular differentiation control, and is a core technology of bio-robotics made up of muscle cells and a polymeric substrate.
Continue reading: Antiviral Activity of Intermetallic Nanoparticles Incorporated into Polymeric Fibers.
Reference
Choi, H., Kim, C., Lee, S. et al. (2021) Nano-sized graphene oxide coated nanopillars on microgroove polymer arrays that enhance skeletal muscle cell differentiation. Nano Convergence, 8. Available at: https://nanoconvergencejournal.springeropen.com/articles/10.1186/s40580-021-00291-6
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.