The potential role of gold nanoclusters (AuNCs) in treatment against the common pathogen Fusobacterium nucleatum has been explored in research published in the journal Frontiers in Materials.
Study: Gold Nanoclusters Exert Bactericidal Activity and Enhance Phagocytosis of Macrophage Mediated Killing of Fusobacterium nucleatum. Image Credit: nobeastsofierce/Shutterstock.com
Engineered nanomaterials and nanotechnologies advancements have provided new systems for antibacterial infectious diseases. Fluorescent imaging has been used to assess the effect of AuNCs on macrophages' capacity to absorb bacteria in both normal and inflammatory conditions.
Using a combination of membrane depolarization and oxidative stress, researchers discovered that gold nanoclusters, AuNCs, had higher antibacterial activity against F. nucleatum in vitro.
Interestingly, macrophages showed increased phagocytosis of F. nucleatum in the existence of AuNCs without causing considerable cytotoxicity. As a result, AuNCs offer a novel platform for preventing and treating F. nucleatum-related illnesses.
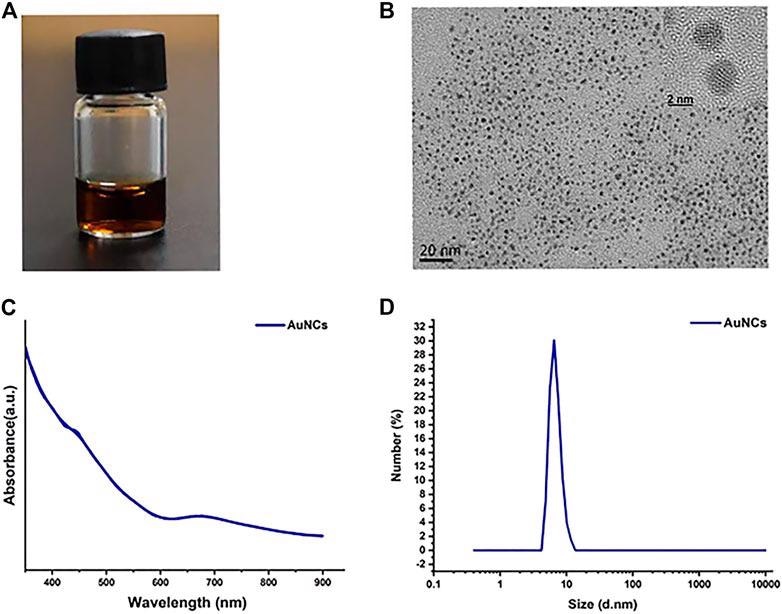
Characterization of AuNCs. (A) A photograph of AuNCs solution. (B) Transmission electron microscopic (TEM) and HR-TEM images. (C) UV-vis absorption spectra. (D) DLS size distribution. © Chen, R., et al. (2021)
Microorganism Cause Diseases
Fusobacterium nucleatum, a Gram-negative anoxic conditions microorganism, has been found in the oral microbes of both healthy and diseased people. An abnormal amount of F. nucleatum in the oral cavity has long been reported to create dysbiotic microbial diversity and immune system response disorders, resulting in infectious diseases.
This pathogen, in particular, has recently received attention due to its strong association with colorectal cancer and bad prognosis; however, the exact mechanisms remain unknown. As a result, it has risen to the top of scientific research, potentially providing a target for the recovery of related diseases. A novel and effective strategy for enhancing antibacterial effects are urgently needed.
Nanotechnology, the Efficient Way to be Antibacterial
Previous research has shown that when the size of nanoparticles (NPs) is reduced to less than 2 nm, they behave as broad-spectrum powerful killers of Gram-positive and Gram-negative microbes. Because of their high biocompatibility, ease of preparation, and complexation, gold nanoclusters (AuNCs) have sparked increased interest.
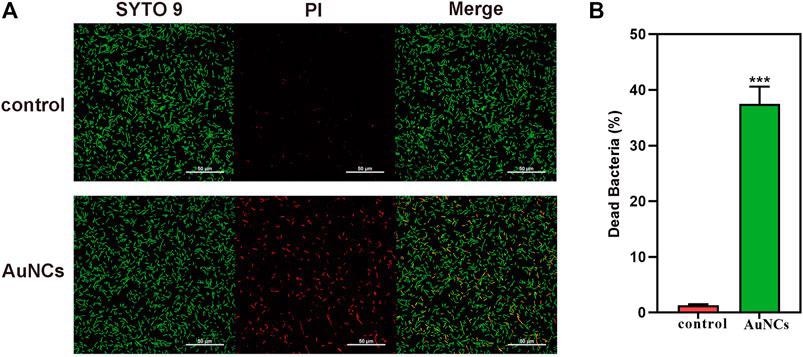
(A) Live/dead fluorescent staining images of F. nucleatum and (B) percentage of dead F. nucleatum after being exposed to AuNCs (0.2 mM) for 6 h (***P< 0.001). © Chen, R., et al. (2021)
Research has linked the antimicrobial property of AuNCs to the plentiful production of reactive oxygen species (ROS), that can cause oxidative stress to fatty acids, DNA, and proteins, resulting in substantial cell damage, intracellular leakage, and death of cells.
Another study found that AuNCs functionalized with quaternary ammonium (QA) salts had antibacterial therapeutic benefits in vivo against MDR bacteria without causing drug resistance or toxic effects.
New Findings of Nanotechnology to Bacteria Efficiency
Researchers discovered that when the levels of AuNCs were 0.2 mM, microbial viability and growth were significantly impacted in a time-dependent manner. The inhibitory activity on bacterial growth was particularly noticeable when the exponentially growing phase was reached at 6h, which could be attributed to the imbalance metabolism caused by nanomaterials therapies. Energy metabolism is required for microorganism survival and growth.
Bacteria are injured when there is low metabolic action, and their growth rate slows significantly, even sometimes stopping. Energy respiration is required for microorganismal survival and growth.
AuNCs were recently discovered to have a potent killing operation for Gram-negative bacteria E. coli at low concentrations (100–150 M) within 30–60 minutes.
Gold Nanoparticles to Treat Microbes
The differences in bacterial sensitivity to AuNCs could be attributed to differences in structure rigidity, membrane concentration, and metabolism behavior.
Gram-negative bacteria had higher binding effectiveness with ultra-small AuNCs than Gram-positive microbes. Furthermore, in low-oxygen environments, anaerobes such as F. nucleatum are using other reduction-potential substrates such as nitrogen as acceptors rather than oxygen, resulting in lower respiratory various metabolic efficiency and less responsiveness to antibiotics than aerobic cells. As a result, AuNCs may provide a new platform for treating F. nucleatum-related infections.
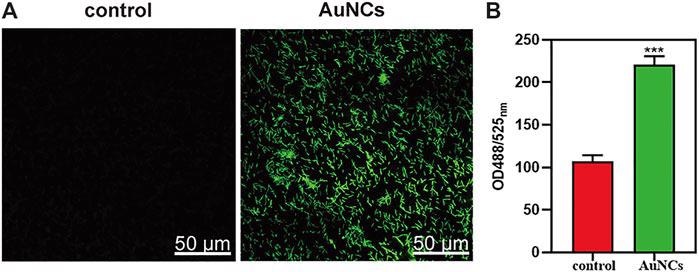
ROS levels of F. nucleatum exposed to AuNCs (0.2 mM) for 2 h. (A) Visualization of ROS concentration in F. nucleatum exposed to AuNCs by confocal microscope. (B) Quantification of ROS fluorescence intensities in F. nucleatum at OD488/525 nm. (***P< 0.001). © Chen, R., et al. (2021)
Antibacterial Activity by Gold Nanoparticle and its Advantage
F. nucleatum was ordinarily a spindle-rod structure with a preserved cellular membrane and distinct characteristics. The bacterium wrinkled and formed nano-sized porous structure on the membrane in the existence of AuNCs, indicating increased membrane fluidity.
Furthermore, the intracellular concentration of treated F. nucleatum was reduced due to broken membrane surface and intracellular content leaking. Based on the findings, it was found that significant ROS augmentation may effectively oxidize the bacterial cell membrane lipid, resulting in large holes in the tissue, which might facilitate much farther internalization and buildup of AuNCs inside bacteria, thereby increasing bacteria killing effectiveness.
Many past studies have looked into the possibility that AuNCs can promote pro-oxidative gene expression. When compared to nanoparticles, atomically accurate AuNCs with much more active sites have efficient catalytic performance that generates excessive ROS for improved antibacterial performance.
Nanotechnology for Infectious Disease Treatment
In the absence of inflammation, macrophages die necrotic, with deficient bacterial killing reactions. In a ROS-mediated reaction, macrophages are assertively induced into strongly phagocytic activity while under high inflammatory conditions.
As a result, by annihilating bacterial membranes and increasing ROS oxidative stress, AuNCs were effective at killing F. nucleatum. However, information on the ROS-generation process is limited and requires further investigation.
Continue reading: Nanoparticle-Based Vaccine Delivery Platform for Infectious Diseases and Cancer
Reference
Chen, R., et al. (2021). Gold Nanoclusters Exert Bactericidal Activity and Enhance Phagocytosis of Macrophage Mediated Killing of Fusobacterium nucleatum. Frontiers in Materials. Available at: https://www.frontiersin.org/articles/10.3389/fmats.2021.803871/full
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.