A novel method to detect Hydrogen Peroxide utilizing TiO2 Nanostructures and ionic liquids was reported in an article published in the journal ACS Omega.

Study: Ionic-Liquid-Stabilized TiO2 Nanostructures: A Platform for Detection of Hydrogen Peroxide. Image Credit: EVANATTOZA/Shutterstock.com
Because of its chemical reactivity, hydrogen peroxide (H2O2) is an essential analyte in the medical world, with most oxidative biochemical processes in living organisms producing H2O2.
The Role of Hydrogen Peroxides
Established peroxidases naturally play an important role in the degradation process of H2O2 in living organisms. They control the concentration of H2O2 and need ambient conditions (temperature and pH) to function. H2O2 can act as a triggering compound, directing various biological processes such as innate immune activation, vascular remodeling, and so on.
The greater the concentration of H2O2 in the cell, however, the more oxidative stress-inducing protein as well as DNA damage occurs. DNA damage tends to increase our vulnerability to the onset of various types of cancer.
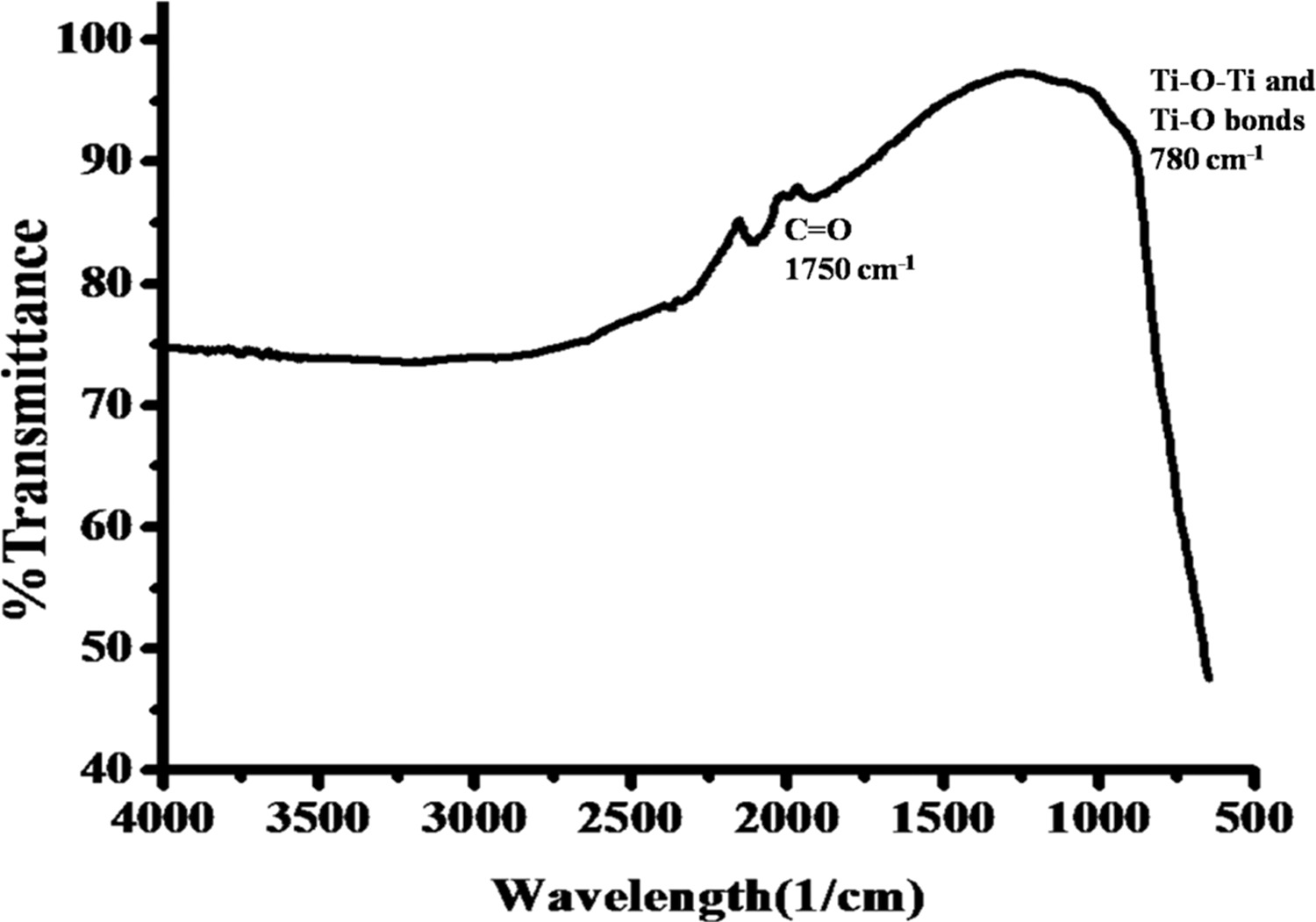
FTIR spectrum of the TiO2 NPs confirming the presence of the Ti–O bond. © Nishan, U., et al. (2021)
Traditional way to Detect H2O2
Numerous strategies for detecting H2O2 have been used over the years, including chromatographic, spectrophotometric, chemiluminescence, and electrochemical techniques. However, some of these methods are harmful to living cells, causing them unsuitable for in situ detection of H2O2 in biological materials.
Some of these methodologies are time-consuming, costly, and complex, restricting their use in laboratories with limited resources. In comparison, detecting H2O2 using colorimetric sensors is a very fast, simple, low-cost, and sensitive technique.
Nanoparticles as a New Path
Nanoparticles are crucial in the development of various types of biosensing. Nanostructures have unique properties due to nano effects such as the quantum effect, higher surface-to-volume ratio, macro-quantum tunneling effect, and surface plasmon resonance (SPR).
As peroxidase mimics, various polymers such as metal oxides, sulfide, and selenide nanomaterials, such as polymer-coated Co3O4 NPs, CeO2 NPs, CuO NPs, V2O5 nanowires, BiFeO3 NPs, CoFe2O NPs, and sheet-like FeS NPs, were used. Moreover, novel ionic liquid-capped TiO2 NPs are recyclable, chemically stable, and extremely efficient, providing excellent detecting and catalytic activity and demonstrating a strong ability to replace expensive noble-metal NPs for biosensing.
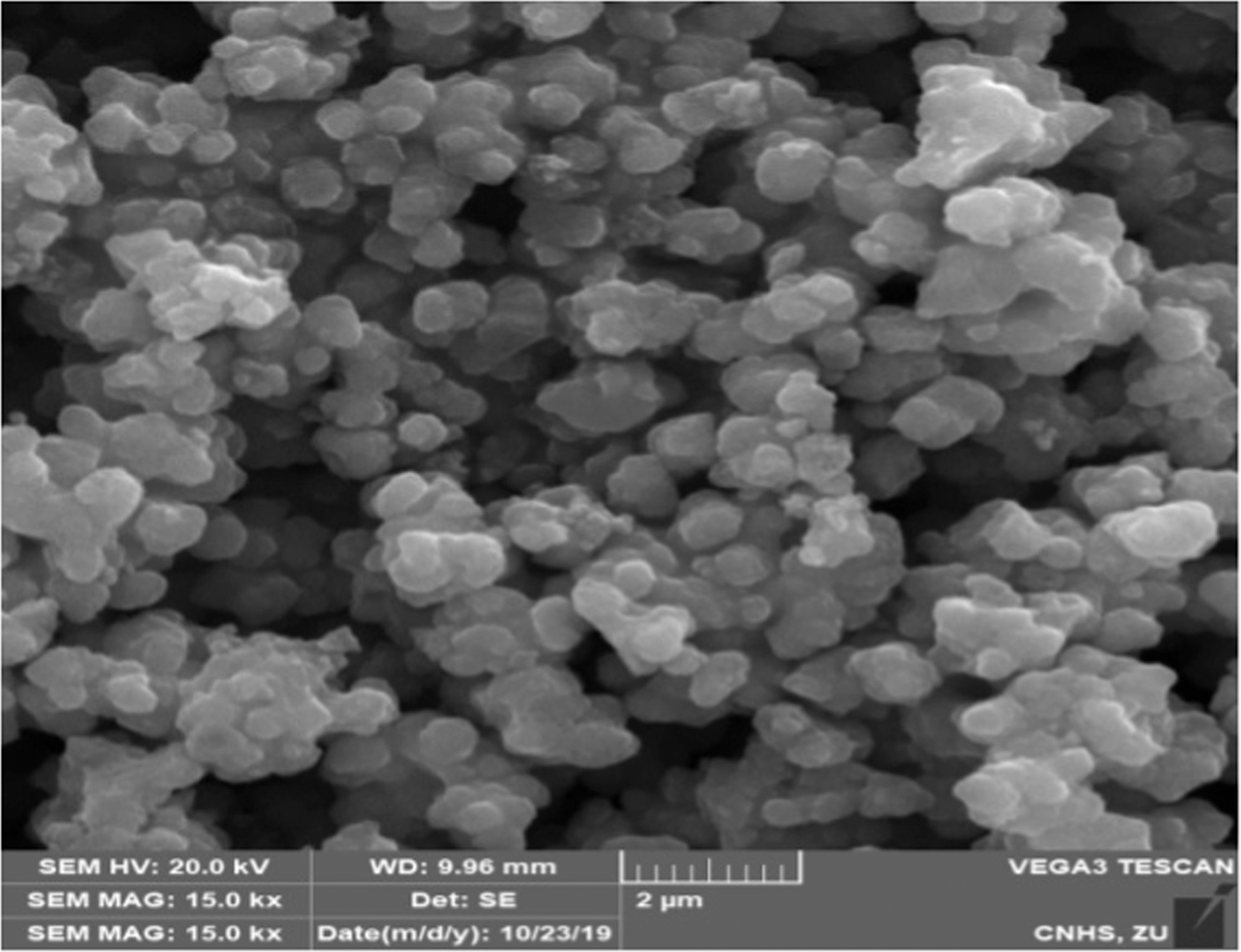
Cross-sectional SEM image of the synthesized TiO2 NPs calcined at 600 °C and 15 000× magnification. © Nishan, U., et al. (2021)
The Research Method Using TiO2 with H2O2 from Cancer Patients
TiO2 nanostructures are most widely utilized in industrial personal care products, medical diagnostics, semiconductors, and photocatalysts. TiO2 photocatalysts have an antibacterial effect as well as the ability to degrade harmful toxins such as superoxide, H2O2, and others.
Because of their high bandgap, nanoparticles based on TiO2 are frequently used on a priority basis in the energy field. Ionic liquids (ILs) are regarded as special chemicals due to their wide heat resistance, negligible vapor pressure, and switchable properties via cation and anion adjustment.
Synthesis Method
TiO2 NPs were synthesized hydrothermally and characterized using various analytical tools such as FTIR, SEM, XRD, TGA, and Raman spectroscopy. To improve sensing activity, the composed NPs were diffused in ionic liquid. The combination of ionic liquid and NPs enhances the system's detecting and catalytic properties.
A simplified, rapid, extremely sensitive, and selective technique for identifying H2O2 has been created, based on the oxidation of chromogenic material by H2O2 in the existence of TiO2 NPs covered with ionic liquid.
Various reaction conditions, such as the quantity of capped NPs; pH; TMB concentration; and time of incubation, have been optimized to achieve the best results of the designed sensor.
The sensitivity and selectivity of the biosensor were also investigated using the aforementioned optimum conditions. H2O2 was detected in urine samples taken from cancer patients.
Research Results Shows TiO2 as a Great Sensor
SEM analysis revealed that the materials had a greater tendency to agglomerate and have a round morphology. The weight reduction at 615 °C is due to the thermal decay of residual organic groups in as-synthesized TiO2. The ionic liquid used stabilizes the NPs and improves their conductivity and enzyme mimic characteristics.
The sensor had a 4 minute incubation time. This same proposed sensor did not produce any results that were influenced by the coexisting organisms in the urine sample. The sensing probe was successfully used to detect H2O2 in urine samples from cancer patients.
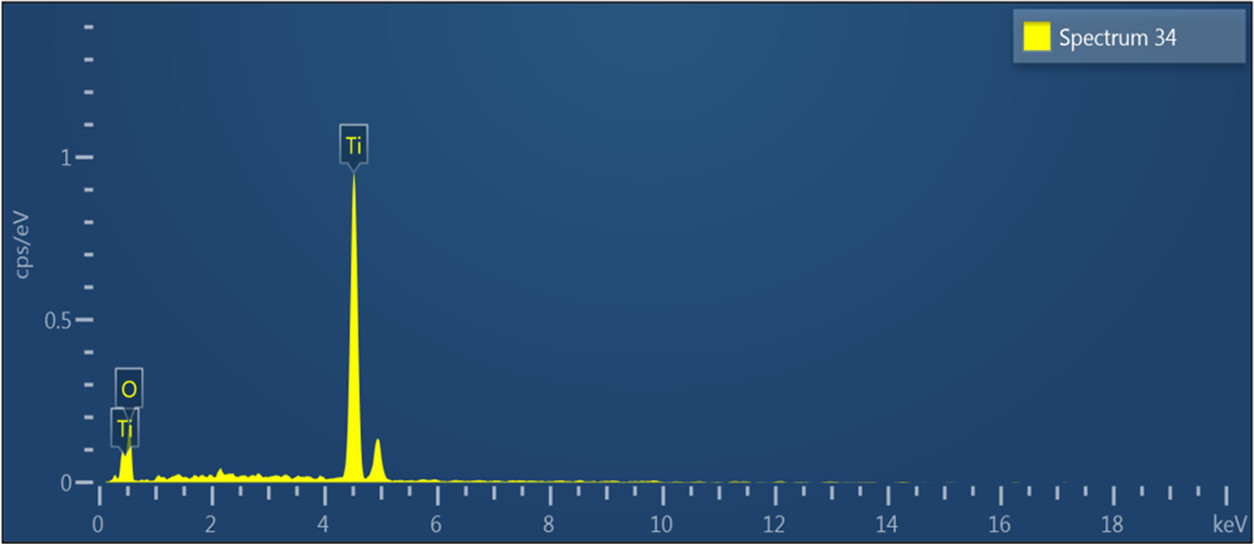
EDX analysis of the prepared TiO2 NPs. © Nishan, U., et al. (2021)
Future Research
Interference tests of the created sensor utilizing multiple nanoparticles are critical to its productivity, which has a wide range of biological applications in clinical diagnosis. Additionally, the sensor's stability was determined by testing the reaction with H2O2 after five months; no significant changes in sensitivity or selectivity were noted.
This experiment proves that the sensor is extremely reliable and repeatable. Exploring other types of nanoparticles may be a future avenue for H2O2 detection technologies.
Continue reading: How do Optical Delay Lines and Delay Line Stages Work?
Reference
Nishan, U., et al. (2021). Ionic-Liquid-Stabilized TiO2 Nanostructures: A Platform for Detection of Hydrogen Peroxide. ACS Omega 2021, 6, 48, 32754–32762. Available at: https://pubs.acs.org/doi/10.1021/acsomega.1c04548
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.