In the latest study published in the journal nanomaterials, nanopowders of Fe2O3 and Fe2O3/zeolite are manufactured for the first time by chemical precipitation employing rusted iron waste and natural zeolite heulandite.

Study: Recycling Rusty Iron with Natural Zeolite Heulandite to Create a Unique Nanocatalyst for Green Hydrogen Production. Image Credit: Quisquilia/Shutterstock.com
Corrosion-induced iron rust is a major source of risk, contamination, and industrial financial crises.
Importance of Hydrogen Energy
Hydrogen is a crucial form of power for multiple reasons, the most significant of which is its abundance. Hydrogen can be produced on-site where it is required, or it can be synthesized and provided remotely.
To produce hydrogen gas, hydrocarbons, petroleum, biodiesel, carbon, or water can all be utilized. Levels of pollution, technical challenges, and energy requirements differ depending on the sources used. It is a non-toxic compound that is rarely used as a fuel source. This means that it is environmentally friendly and does not harm public health.
Introduction to Fe2O3
Various semiconducting metals and compounds have been employed to enhance photoelectrochemical (PEC) performance. Because of its strong absorption, outstanding chemical durability, high efficiency, and huge availability, Fe2O3 is utilized as a photocatalyst for the PEC. Furthermore, it is a non-toxic and environmentally friendly chemical, all of which are essential for cost-effective large-scale solar energy conversion.
Limitations of Fe2O3
Although Fe2O3 has many advantages for its use in the photoelectrical industry, it also has many disadvantages that restrict its implementation in functional photocatalytic applications. Examples include lower absorption extents of holes, inadequate permeability, fast electron-hole rearrangement, inadequate adsorbent property, and difficulty in recovery.
Several investigations immobilized the Fe2O3 nanoparticles on various supports such as activated carbon, clay, and zeolite to circumvent these drawbacks. Zeolite is one of the most researched substances among these.
Advantages of Zeolite
Apart from its semiconducting properties, zeolite has grabbed the curiosity of academics due to its strong adsorption capability against organic pollutants.
Zeolite has excellent electrostatic interaction characteristics, making it ideal for the adsorption/degradation of natural pigments. It also features a large number of distinct regions, variable hydrophobicity/hydrophilicity, and photocatalytic durability. Furthermore, zeolite is inexpensive, plentiful, and non-toxic.
Zeolite is a monocrystal mineral comprised of tetrahedral Si and Al atoms. It has a wide range of uses, including concrete, ceramics, semiconductors, and wastewater division for hydrogen evolution. In recent years, zeolite has been employed as a substrate for semiconducting PEC enzymes to increase the rate of hydrogen generation.
When iron comes into touch with moisture in the air, it corrodes. Millions of tons of rusty debris are produced due to the corrosion of iron buildings.
As a result, given the widespread usage of iron wires over the world, recycling/reusing rusty debris is expected to significantly reduce waste levels. This study aimed to substitute iron precursors with rust wastes as a source of iron for the chemical precipitation production of Fe2O3 and Fe2O3/zeolite nanopowder.

Schematic of the synthesis steps of Fe2O3/zeolite. © Shaban, M., Sabt, M. B., Ahmed, A. M. & Mohamed, F., (2021).
Research Findings
XRD was used to determine the crystallinity and phase of Fe2O3, zeolite, and Fe2O3/zeolite nanocomposite. The strong and intense peaks suggested that the hematite nanoparticles produced with bulk Fe-based rust were of great purity and crystallinity. These XRD results were comparable to previously generated iron oxide in a variety of works utilizing synthetic precursors.
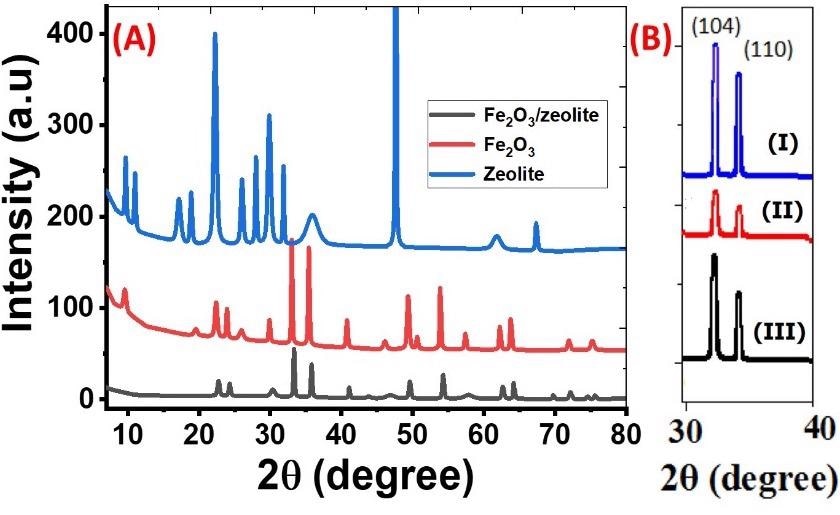
(A) XRD patterns of zeolite, Fe2O3, and Fe2O3/zeolite nanocomposite; and (B) XRD (104) and (110) peaks of Fe2O3 (I), Fe2O3 (II), and Fe2O3 (III). © Shaban, M., Sabt, M. B., Ahmed, A. M. & Mohamed, F., (2021).
The primary core characteristics of Fe2O3 and Fe2O3/zeolite XRD patterns were remarkably similar, demonstrating that the addition of zeolite did not affect the structural properties of the Fe2O3 photosensitizer. However, coupling Fe2O3 with zeolite increased the FWHM and resulted in a minor shift in the plane location of the Fe2O3 toward higher angles following coupling.
The catalytic efficiency of a photocatalyst is widely recognized to be substantially linked to its surface shape. SEM pictures of natural zeolite revealed micro/nano-stones in various non-uniform sizes and shapes.
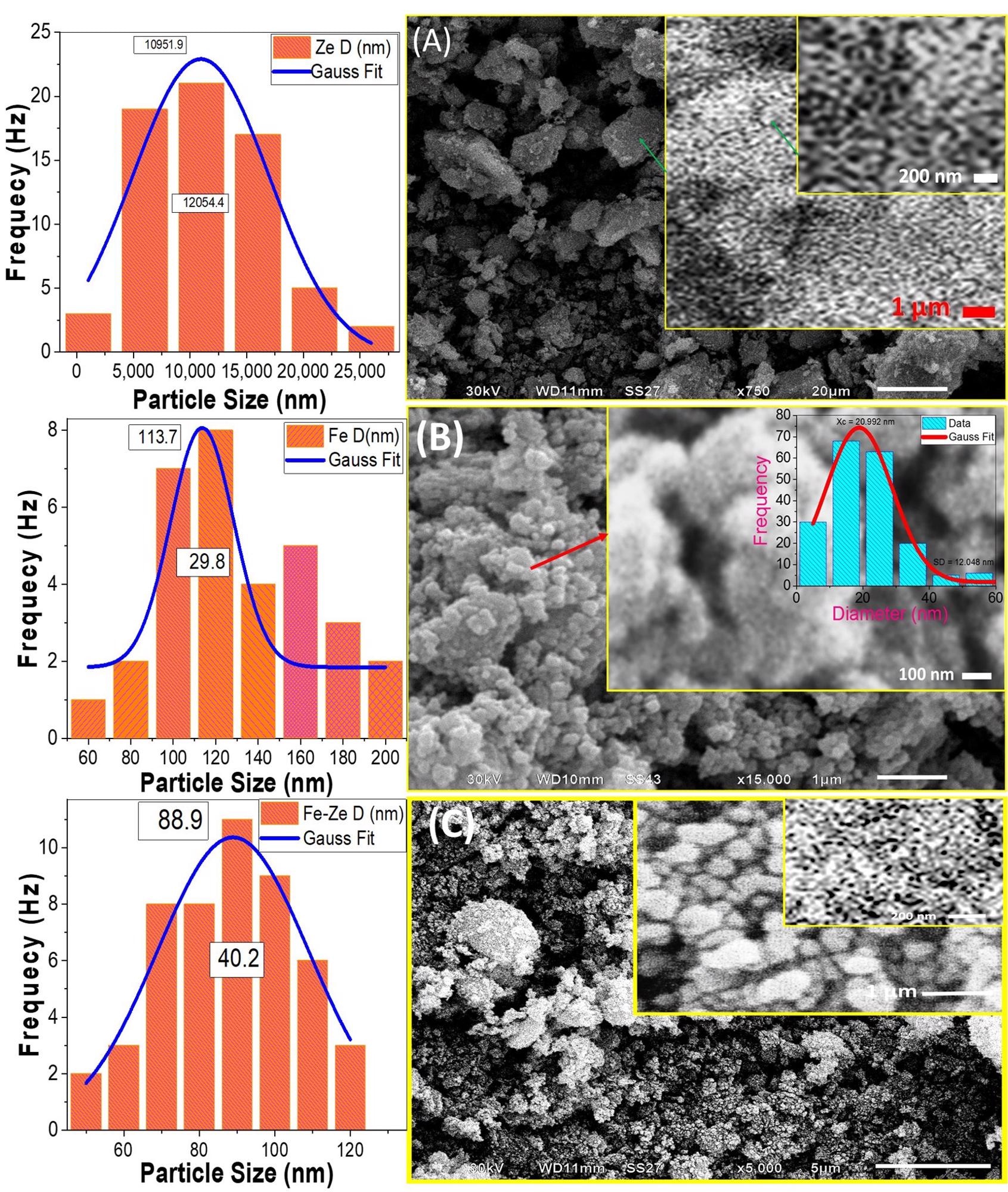
SEM micrographs and the corresponding particle size distribution for (A) natural zeolite, (B) Fe2O3, and (C) Fe2O3/zeolite. The inset of (B) shows the pore diameter distribution. © Shaban, M., Sabt, M. B., Ahmed, A. M. & Mohamed, F., (2021).
PEC activity is predicted to benefit from the interaction of Fe2O3 nanoparticles and their deposition over the zeolite. Furthermore, reducing the size of the particles to the nanoscale and increasing the pores might provide a large effective surface area of the Fe2O3 nanocatalyst. This can provide a high level of absorption of incident light.
The durability of the Fe2O3/zeolite photocatalytic activity for H2 production is investigated in 0.9 M KOH for an extended period under white light and a supply voltage of +1 V. Within the first 16 seconds, the J-value plummeted substantially. There is a modest decrease in J-value for duration > 16 s before reaching a stable value of around 4.63 mA/g for 60 s. This demonstrated very high stability and a lengthy photochemical life expectancy, validating their extended H2 production lifetime.
In summary, a very successful recycling methodology for rusted iron wastes, as well as a modular process for the synthesis of Fe2O3 and Fe2O3/zeolite nanocomposite, have been disclosed. Researchers demonstrated lower dimensions, opening the door to potential future uses.
Continue reading: Game-Changing Electrocatalyst and the Future of Large-Scale Clean Energy.
Reference
Shaban, M., Sabt, M. B., Ahmed, A. M. & Mohamed, F., (2021). Recycling Rusty Iron with Natural Zeolite Heulandite to Create a Unique Nanocatalyst for Green Hydrogen Production. Nanomaterials, 11(12). 3445. Available at: https://www.mdpi.com/2079-4991/11/12/3445#
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.