In a study published in the International Journal of Environmental Research and Public Health, using a basic mix of graphene and multi-walled carbon nanotubes (MWCNTs) as an electrode paste, simple electrolytic approaches for DCF measurement based on voltammetry and amperometry methodologies were developed.
Study: Electrochemical Method for Ease Determination of Sodium Diclofenac Trace Levels in Water Using Graphene—Multi-Walled Carbon Nanotubes Paste Electrode. Image Credit: Dimarion/Shutterstock.com
Pharmaceutical Products are Polluting Water
Reliable live tracking based on modern measuring technologies is critical to comprehending and addressing the problem of water quality control, particularly pharmacological pollution in water.
Medicines administered to people or pets are expelled in the form of feces and urine, with up to 90% of oral dosages expelled as active chemicals.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a kind of pharmaceutical and personal care products (PPCPs) and include a category of drugs with varying chemical makeups and medicinal capabilities that share at least three typical characteristics: core medicinal characteristics, the fundamental mode of action, and harmful impacts.
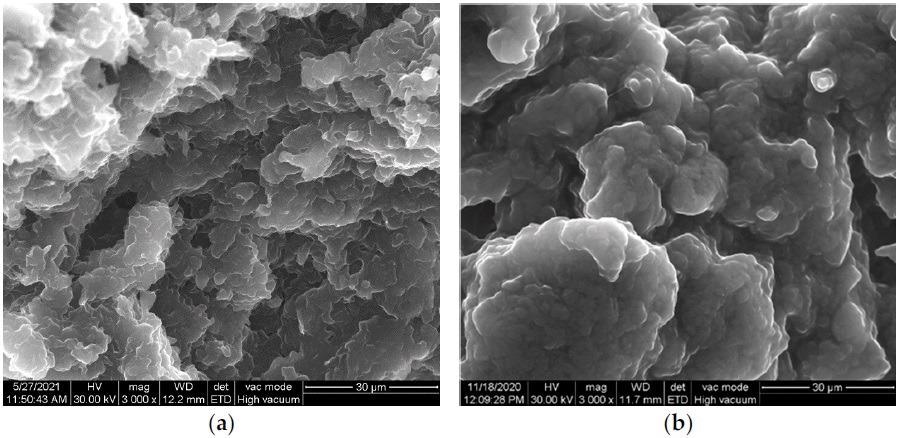
SEM images of paraffin oil-based carbon paste: (a) CNT and (b) GR-CNT. © Motoc, S., Manea, F., Baciu, A., Orha, C., & Pop, A. (2021)
Diclofenac sodium (DCF) is a derivative of arylalkanoic acid that is extensively utilized to treat chronic diseases and swelling. It may be acquired without the need for a doctor's prescription, allowing its usage and implicating its significant possibility for environmental contamination.
Its strong polarity and wettability allow simple flow of water, and its abundance in groundwater resources may be fairly significant.
Inadequate sewerage and water waste treatment are other major contributors to the ecosystem's drugs. DCF is expelled by the user, either in a home location or in a clinic, and is dumped into sewage before being sent to wastewater treatment plants (WWTPs). Because WWTPs are unable to entirely eliminate such medications during wastewater treatment, they are found in WWTP waste and may be transferred into surface water.
DCF Detection via Electrochemistry
Given their potentially harmful influence on the ecosystem and on human wellbeing, sophisticated analytical methodologies for DCF trace identification are necessary.
For the estimation of DCF, a broad range of detecting techniques have been devised. Electrolytic diagnosis provides the benefits of greener chemical analysis by using electrons as an eco-friendly agent, as well as excellent live system surveillance.
Electrolytic solutions provide benefits such as high sensitivity and specificity, a large linear range of concentrations, inexpensive equipment, a small footprint, and minimal energy usage.
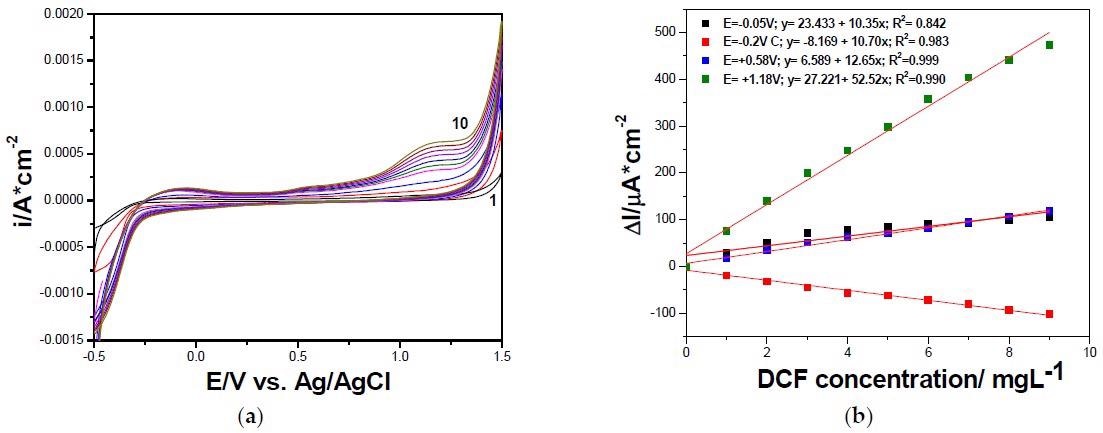
(a) Cyclic voltammograms recorded at GR-CNT paste electrode with the scan rate of 0.050 V·s−1 in 0.100 M Na2SO4 supporting electrolyte (curve 1), and DCF concentrations ranged from 1.00 to 9.00 mg·L−1 (curve 2–10). (b) Calibrations plots of peak current vs. DCF concentrations at the potential value: E = –0.050 V vs. Ag/AgCl (anodic), E = +0.580 V vs. Ag/AgCl (anodic), E = +1.180 V vs. Ag/AgCl (anodic), and E = –0.200 V vs. Ag/AgCl (cathodic). © Motoc, S., Manea, F., Baciu, A., Orha, C., & Pop, A. (2021)
Carbon-based electrodes having a nanoscale structure must be modified to match scanning performance criteria to build an extremely precise electrolytic detecting technique. For DCF identification, NC has been augmented with a variety of sophisticated elements, including metallo-organic architecture, copper, ionic liquids, chitosan–copper complexes, and Au–Pt bimetallic nanoparticles.
Creating nanostructured carbon paste electrodes is simple for building an electrolytic identification technique of DCF due to its properties.
In fact, the paste content of the electrode may be readily varied by simply combining carbon with various oils and altering components without the use of a template or framework. The combination of graphene with carbon nanotubes (CNTs) was examined in this work to create enhanced electrolytic identification of trace-level DCF in water.
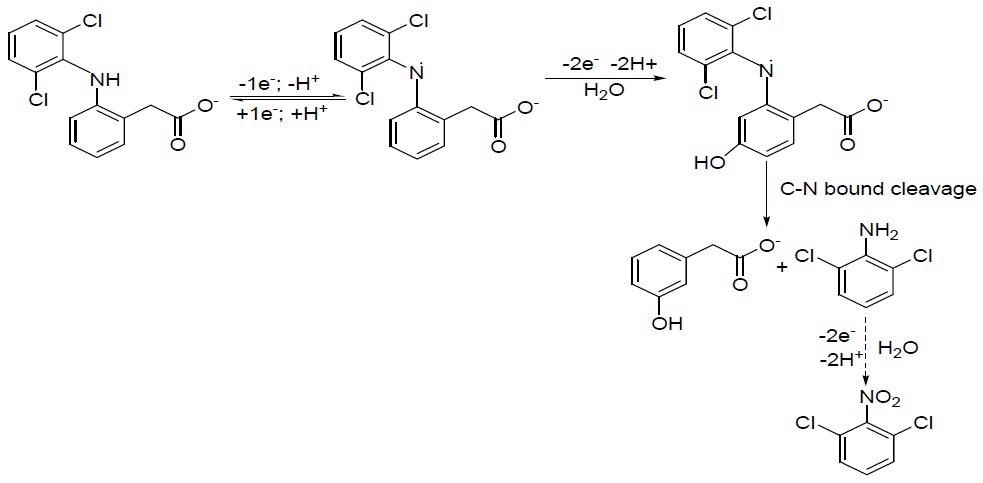
A proposed mechanism of DCF oxidation. © Motoc, S., Manea, F., Baciu, A., Orha, C., & Pop, A. (2021)
Key Findings of the Study
In contrast with MWCNT paste electrodes, facile incorporation of graphene into MWCNT paste electrode (GR-CNT) resulted in a steady and stronger electrolytic performance owing to increased electroactive contact area.
Cyclic voltammetry was used to characterize the electrolytic response of diclofenac on CNT and GR-CNT electrodes, and the voltammetry response of the GR-CNT electrode was superior.
A comprehensive analysis of the scanning rate effect on the cyclic voltammetry morphologies associated with the DCF content ranges revealed a highly complicated process of DCF oxidation on the GR-CNT electrode. The autocatalysis effect of absorption and adsorption discovered at low DCF concentrations enabled the electroanalytical outputs of sophisticated voltammetry and amperometry tailored for DCF identification to be improved.
Furthermore, the same procedure was followed before optimizing multiple-pulsed amperometry, which was confirmed in actual tap water for DCF identification.
GR-CNT demonstrates high usefulness for real-world implementation in creating a scanning and surveillance technique for determining DCF in the water (WWTP effluent, surface water, and drinking water) consumption cycles.
Continue reading: Replenishing Water Sources with Electrospun Nanofiber Membranes.
Reference
Motoc, S., Manea, F., Baciu, A., Orha, C., & Pop, A. (2021). Electrochemical Method for Ease Determination of Sodium Diclofenac Trace Levels in Water Using Graphene—Multi-Walled Carbon Nanotubes Paste Electrode. International Journal of Environmental Research and Public Health, 19(1). Available at: https://www.mdpi.com/1660-4601/19/1/29
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.