An innovative method converts nanoparticles into simple reservoirs for the storage of hydrogen.
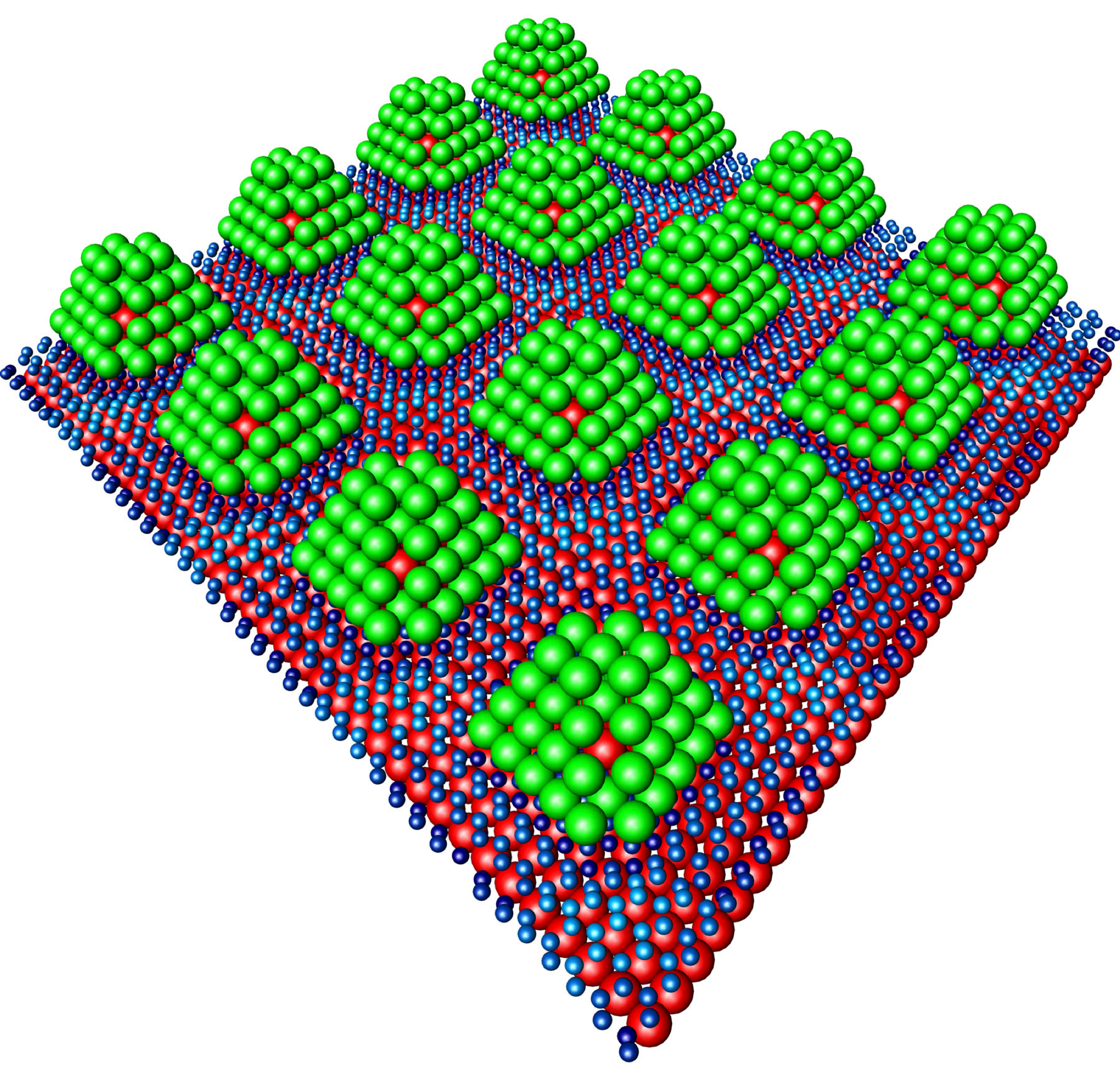 The palladium nanoparticles (green) are stabilized by a core of iridium (red). Hydrogen can accumulate on their surface like a kind of chocolate glaze - and can be released again by heating. Image Credit: DESY, Andreas Stierle.
The palladium nanoparticles (green) are stabilized by a core of iridium (red). Hydrogen can accumulate on their surface like a kind of chocolate glaze - and can be released again by heating. Image Credit: DESY, Andreas Stierle.
The highly volatile gas is a hopeful energy carrier for the future, which could offer climate-friendly fuels for lorries, ships, and airplanes, for instance, and enabling climate-friendly steel and cement production.
However, storing hydrogen is expensive: either the gas must be preserved in pressurized tanks, at up to 700 bar, or liquefied. This implies cooling it down to −253 °C. Both the procedures add up extra energy.
Research headed by DESY’s Andreas Stierle has laid the foundations for an alternative technique: storing hydrogen in small nanoparticles made of the valuable metal palladium, just 1.2 nm in diameter. The fact that palladium can absorb hydrogen like a sponge has been well-known for some time.
However, until now getting the hydrogen out of the material again has posed a problem. That’s why we are trying palladium particles that are only about one nanometre across.
Andreas Stierle, Head, NanoLab, Deutsches Elektronen Synchrotron
To guarantee that the small particles are adequately sturdy, they are stabilized by a core made of the rare useful metal iridium, and are fixed to a graphene support - an extremely thin layer of carbon.
We are able to attach the palladium particles to the graphene at intervals of just two and a half nanometers. This results in a regular, periodic structure.
Andreas Stierle, Head, NanoLab, Deutsches Elektronen Synchrotron
The team also includes scientists from the Universities of Cologne and Hamburg, and published its study findings in the American Chemical Society (ACS) journal ACS Nano.
DESY’s X-ray source PETRA III was utilized to observe what happens when the palladium particles come in touch with hydrogen: Basically, the hydrogen fits in the nanoparticles’ surfaces, with barely any of it penetrating inside.
The nanoparticles could be pictured as resembling chocolates: an iridium nut at the center, enclosed in a layer of palladium, instead of marzipan, and chocolate-coated on the exterior by the hydrogen.
All one should do to retrieve the stored hydrogen is for a small amount of heat to be added; the hydrogen is quickly discharged from the surface of the particles since the gas molecules do not have to exit from inside the cluster.
Next, we want to find out what storage densities can be achieved using this new method.
Andreas Stierle, Head, NanoLab, Deutsches Elektronen Synchrotron
But few challenges exist which should be overcome before proceeding to practical applications. For instance, other forms of carbon structures may be a more appropriate carrier compared to graphene. The experts considered using carbon sponges, consisting of small pores, where significant amounts of the palladium nanoparticles must fit inside.
Journal Reference:
Franz, D., et al. (2021) Hydrogen Solubility and Atomic Structure of Graphene Supported Pd Nanoclusters. ACS Nano. doi.org/10.1021/acsnano.1c01997.